Biomed Res Bull. 2(4):172-177.
doi: 10.34172/biomedrb.2024.23
Original Article
Glatiramer Acetate Effect on the Expression of MiR-21 and Let7i Via Down-Regulation of MAPK and JAK Signaling Pathways in Stroke Mice Model and PBMCs of Stroke Patients
Milad Golizadeh 1  , Nima Sadeghi 2, Paria Kahnamouyi 2, Reza Salehi 1, *
, Nima Sadeghi 2, Paria Kahnamouyi 2, Reza Salehi 1, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Neuroscience Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Ischemic stroke is a prominent neurodegenerative disease, characterized by a decrease in the body’s functional abilities. It is proposed that many immunity members and cells might play a role in the pathogenesis of stroke, among which microRNAs have recently attracted much attention. MicroRNAs are noncoding RNAs involved in various immune functions and disorders. The present study aimed to evaluate the effect of Glatiramer acetate on microRNAs in stroke through immune signaling pathways.
Methods:
In this study, photothrombotic (PT) stroke was induced in adult C57BL/6J mice. After three days of recovery, the mice were treated with Glatiramer acetate on day three post-stroke. Simultaneously, the peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples of the stroke patients. The expression of miR-21 and Let7i was evaluated using real-time polymerase chain reaction (PCR). Additionally, the expression of MAPK and JAK pathways was assessed by Western blot. The P value of 0.001 was considered statistically significant.
Results:
Glatiramer acetate decreases the expression of let-7i and miR-21 in both the stroke mice model and PBMCs of stroke patients, compared to controls. Furthermore, Western blot analysis showed that Glatiramer acetate inhibits the immune signaling pathways involving MAPK and JAK in the stroke mice model.
Conclusion:
In addition to its known neuroprotective effects during the acute phase of experimental stroke, Glatiramer acetate administration decreases the expression of miR-21 and let-7i via down-regulation of immune signaling pathways.
Keywords: Glatiramer acetate, MiR-21, LET7i, Stroke, MAPK, JAK, Immune signaling pathway
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
Ischemic stroke is a neurodegenerative disease that is considered the second leading cause of mortality and dementia, posing a significant health burden. The pathophysiology of ischemic stroke is believed to be dependent on inflammation. Innate immune cells such as microglia and macrophages are activated instantly after ischemia and produce tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), leading to post-ischemic inflammation.1-3 Adaptive immune cells, including B cells and Th0 (naive CD4 + helper) lymphocytes, also contribute to the inflammatory process. Overall, microglial/macrophage cells and type-1 helper cells (Th1) are considered harmful, whereas Th2 and regulatory T cells (Treg) have protective roles. Given the importance of the immune system and neuroinflammatory response in ischemic stroke pathophysiology, evaluating the efficacy and safety of particular immunomodulatory agents in stroke treatment is crucial.4,5
Glatiramer acetate (GA), or Copolymer-1 (Cop-1), is a synthetic peptide composed of four amino acids (i.e., alanine, lysine, glutamic acid, and tyrosine) with immune-modulatory properties.6 GA works by inhibiting Th1 pro-inflammatory cytokines (IFN-γ), inducing T cell activation, decreasing microglial/monocyte reactivity, reducing TNF-α and IL-12 levels, and enhancing IL-10 and transforming growth factor-ß (TGF-ß)7.
MicroRNAs (miRNAs) are non-coding RNA molecules that play a crucial role in regulating various cellular and developmental procedures, including immune cell differentiation, immune response outcomes, cellular proliferation, and apoptosis. More than 20% of miRNAs are found to be altered during the ischemic brain process, indicating that miRNAs are potential mediators in the pathogenesis of ischemic stroke.8-12 MiR-21 is one of the ischemic-related miRNAs with anti-apoptotic and pro-survival effects. MiR-21 also plays a role in regulating angiogenesis, mediated by endothelial cells (ECs). Overexpression of miR-21 can protect against ischemic neuronal death.10,13 The neuroprotective effect of miR-21 is probably mediated through the downregulation of Fas ligand (FasL), a member of the TNF-α family that induces cell death. These results imply that miR-21 has neuroprotective properties in ischemic brain injury and could be a feasible target for ischemic stroke therapy by regulating many associated genes.14-17
Let7i is another miRNA involved in mediating toll-like receptor signaling in monocytes, as well as influencing the differentiation process of dendritic cells and guiding T-cells towards a Th1 phenotype in stroke. Moreover, the let-7i molecule present in the ECs helps prevent endothelium-mesenchymal transition (endMT), which in turn can regulate the inflammatory response among these cells. Interestingly, the expression of let-7 in ECs plays a crucial role in preserving the structural integrity of the brain vasculature. In acute ischemic stroke, this miRNA might regulate the gene expression of leukocytes,18-21 which are involved in mediating immunological responses, leukocyte extravasation, and thrombus formation. These genes and pathways are critical in ischemic stroke. It has been demonstrated that let-7i may also exert regulatory effects on thrombin, a key coagulation factor in ischemic stroke. Investigating the interplay between GA and these miRNAs might yield valuable insights into its therapeutic efficacy in stroke.22 This study aimed to examine the impact of GA on the expression levels of miR-21 and let-7i in animal models of stroke, as well as in blood samples obtained from individuals with a history of stroke. It further strives to provide a fresh perspective on stroke management and the potential development of therapeutics based on miRNAs.
Materials and Methods
Photothrombotic Stroke Model
All procedures were conducted in accordance with the guidelines set by the Tabriz University of Medical Sciences Research Council for animal studies (Ethical ID number: IR.TBZMED.VCR.REC.1400.191). The study used 11-week-old BALB/c mice (40-50 g). Mice were housed in propylene cages in animal housing facilities with an ambient temperature of 25 ± 2°C and relative humidity of 45%-55%. They were kept on a 12-hour light/dark cycle, with ad libitum access to pellet chow and water. The mice were divided into two groups (n = 10 animals in each group): (a) Photothrombotic (PT) stroke group with vehicle (saline) treatment as a control group and (b) PT stroke group with GA treatment. Mice were anesthetized using 4% isoflurane and maintained under isoflurane with 1%-1.5% isoflurane in a mixture of nitrous oxide (70% v/v) and oxygen (30% v/v) throughout the surgical process. Rose Bengal (100 mg/kg body weight, Sigma, USA) was injected intraperitoneally five minutes before illumination. The skull was exposed and cleaned, and a fiber optic cable delivering cold light (6 mm diameter) was placed on the skull using a stereotaxic frame. To reduce the scattering of the laser light, a sterile mask was placed near the primary somatosensory cortex. A green laser (532 nm) was used to photoactivate the Rose Bengal, illuminating the S1FL for 20 minutes (average power density was 23 mW), with the laser positioned 3 cm above the skull. Before suturing, Marcaine (2 mg/kg, administered at 1.67 mg/mL in saline) was applied to the incision site, and saline (0.01 mL/g) was administered subcutaneously after suturing. Animals were monitored in individual cages during recovery and were given additional subcutaneous saline (0.25 mL) four hours post-surgery. Inclusion criteria for the stroke treatment group included any sign of ipsilateral forelimb weakness post-stroke, while exclusion criteria included absence of forelimb weakness, signs of contralateral forelimb weakness, inability to walk, or circling while walking. Subsequently, 24 hours post-stroke, the mice were administered either GA (0.5 mg/kg) or saline (i.p.), with doses repeated every other day for up to 28 days.
Peripheral Blood Mononuclear Cells From Stroke Patients
Twenty ischemic stroke patients were also included in the present study. The inclusion criteria were stroke patients without any other acute diseases or infections. The exclusion criteria were patients with acute or autoimmune diseases. Whole blood was collected from these patients, and peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque (FicollTM Paque Plus; GE Healthcare). The cells were cultured in RPMI-1640 medium (Shanghai BioSun SciTech Co., Ltd.) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences) and 1% penicillin-streptomycin (Beijing Leagene Biotech Co., Ltd.) and incubated at 37 °C with 95% humidified air and 5% CO2. After 24 hours of culture, cell morphology was observed under an inverted microscope. The cells were treated with GA after 48 hours of culturing.
Reverse Transcription-Quantitative Polymerase Chain Reaction
To evaluate miR-21 and let-7i expression, total RNA was isolated from tissues and cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.). One microgram (1 µg) of RNA was reverse transcribed to obtain cDNA using the RevertAidTM cDNA Synthesis kit (Thermo Fisher Scientific, Inc.), with reaction conditions set at 85 °C for five minutes. Quantitative polymerase chain reaction (qPCR) was performed using an ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and iTaqTM Universal SYBR®-Green (Bio-Rad Laboratories, Inc.). The qPCR protocol was as follows: initial denaturation at 94 °C for five minutes, followed by 30 cycles of denaturation at 94 °C for 30 seconds, annealing at 65 °C for 30 seconds, and extension at 70 °C for 30 seconds. Beta-actin was used as the control.
Western Blotting
Mice were surrendered after anesthesia, and the peri-infarct cortical tissues were harvested for analysis. Samples were lysed on ice using protein extraction reagents (Santa Cruz, USA) for one hour, then centrifuged at 12 000 rpm at 4 °C for 10 minutes. The supernatant was collected and stored at −20 °C. Protein concentrations were quantified using the Bradford protein assay kit (Bio-Rad, California, United States). Samples were mixed with 5 × loading buffer and boiled for 10 minutes for denaturation. Twenty micrograms of the total protein were loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, then transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk in Tris-buffered saline with Tween 20 (TBST) at room temperature and incubated with primary antibodies against GAPDH, MAPK, and JAK at 4 °C overnight. The membranes were washed three times with TBST and then incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for one hour.
Statistical Analysis
IBM SPSS Statistics (version 23, IBM, SC, USA) was utilized for statistical analysis. Data were presented as mean ± standard deviation. For multiple comparisons, one-way analysis of variance (ANOVA) with a t-test was used. A P value < 0.05 was considered statistically significant.
Results
The Effect of Glatiramer Acetate on the Expression of Let-7i and MiR-21 in the Stroke Mice Model
To determine the expression of let-7i and miR-21 in stroke mice model tissue, total RNA was extracted, and RT-PCR was conducted. The results revealed that GA significantly decreased the expression of let-7i and miR-21 relative to the control mice group (Figure 1).
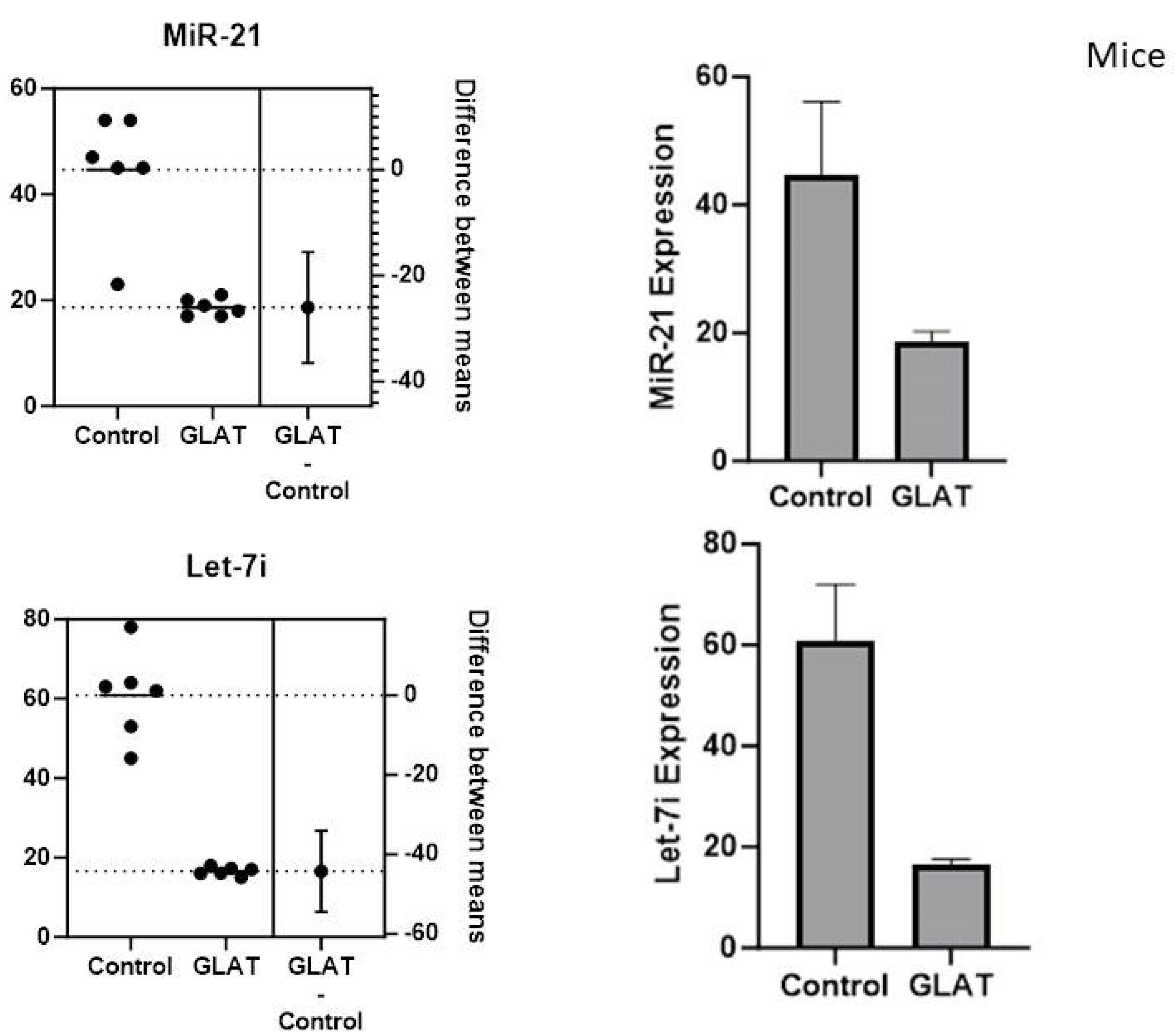
Figure 1.
The Expression of MiR-21 and Let-7i in the Stroke Mice Model
.
The Expression of MiR-21 and Let-7i in the Stroke Mice Model
The Effect of Glatiramer Acetate on the Let-7i and MiR-21 in Peripheral Blood Mononuclear Cells of Stroke Patients
The expression of let-7i and miR-21 in PBMC-isolated cells from stroke patients showed that GA administration diminished the expression of let-7i and miR-21 expression in treated cells compared to untreated cells (Figure 2).
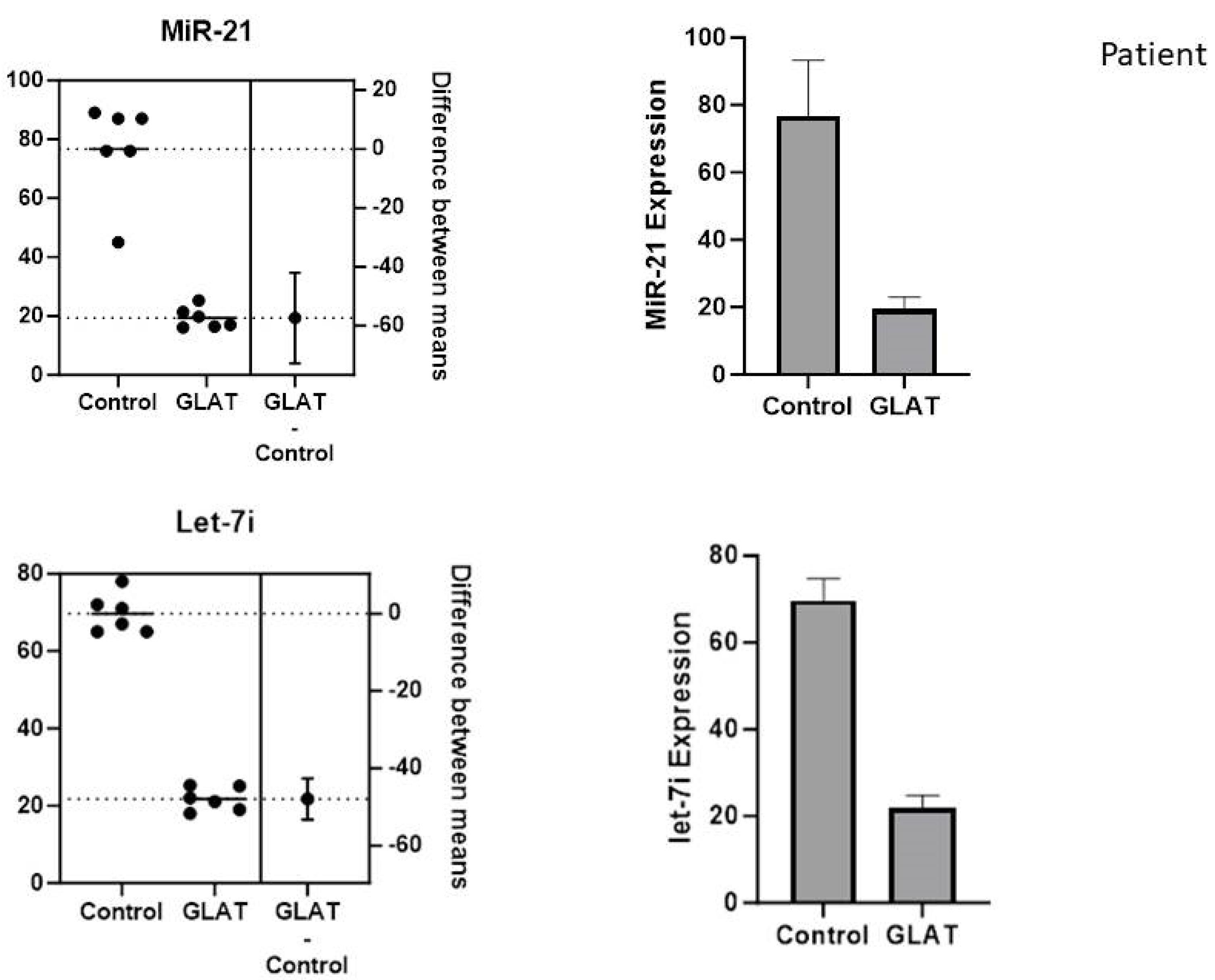
Figure 2.
The Downregulation of MiR-21 and Let-7i Expression in PBMCs of Stroke Patients. Note: PBMCs, peripheral blood mononuclear cells
.
The Downregulation of MiR-21 and Let-7i Expression in PBMCs of Stroke Patients. Note: PBMCs, peripheral blood mononuclear cells
Downstream MAPK and JAK Proteins Under Western Blot Analysis
The downstream proteins MAPK and JAK were analyzed by Western blot analysis. As shown in Figure 3, the expression of MAPK and JAK was significantly lower in stroke mice models treated with GA compared to untreated stroke mice models (P < 0.01 and P < 0.01, respectively). Western immunoblot bands were quantified using a Bio-Rad calibrated densitometer (GS-800) with the vendor’s software (Bio-Rad Laboratories), and GAPDH served as the internal control for all analyses.
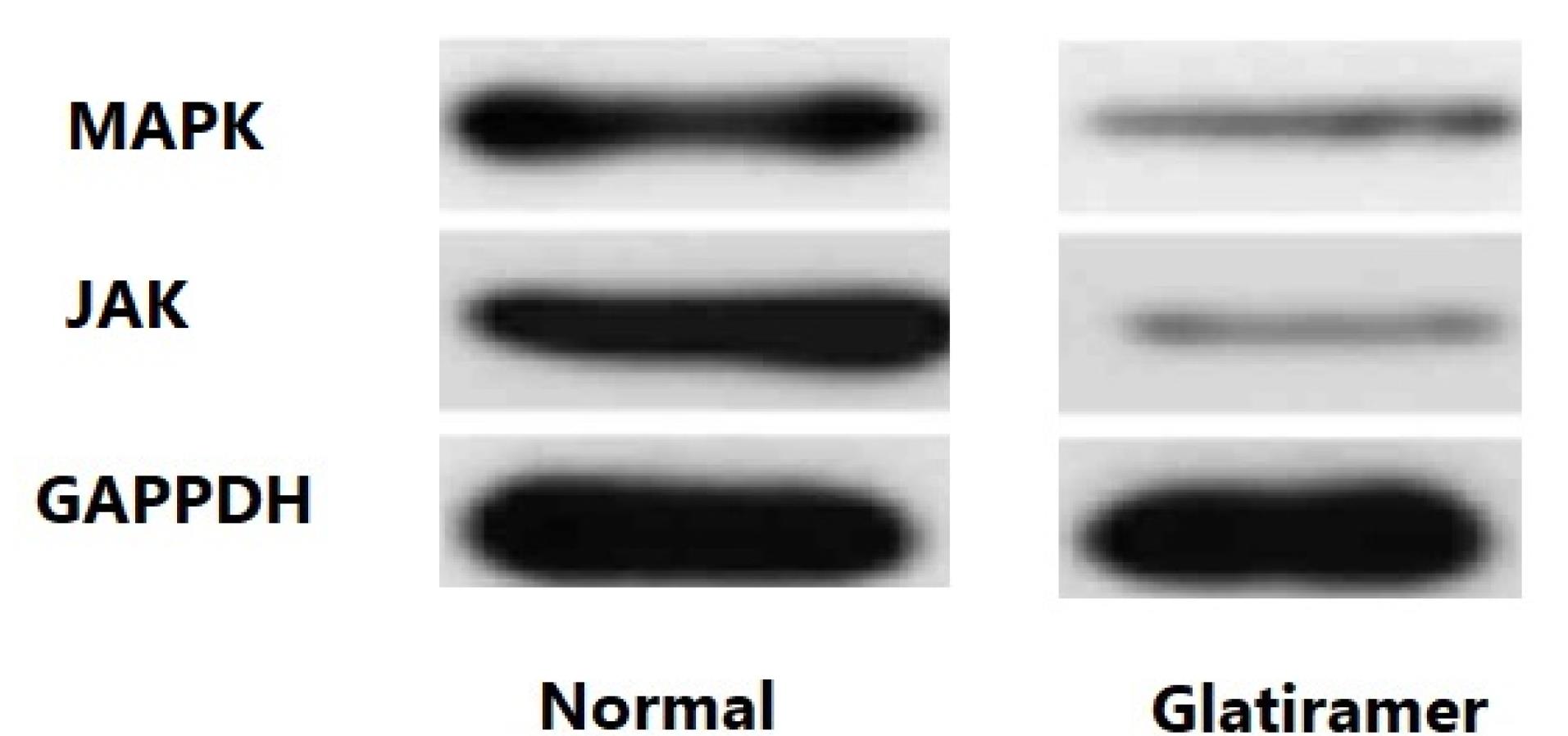
Figure 3.
The Expression Levels of MAPK and JAK Proteins in the Stroke Mice Model
.
The Expression Levels of MAPK and JAK Proteins in the Stroke Mice Model
Discussion
Many Studies have suggested that several miRNAs regulate essential target genes implicated in stroke. MiR-21 is one of the well-known miRNAs that play a significant role in the etiology of various types of stroke.22 Previous studies on ischemic stroke have indicated that miR-21, a crucial miRNA, promotes survival and inhibits apoptosis during the development of cerebral ischemia, making it a valuable biomarker for diagnosis or a target for treatment.23,24 According to a study by Zhou et al, miR-21 largely prevented N2a neuroblastoma cells from undergoing apoptosis after being subjected to oxygen-glucose deprivation and reoxygenation (OGD/R).25 Buller et al found that OGD/R-induced apoptosis was reduced in embryonic neurons cultured in vitro when miR-21 was overexpressed.26 Another investigation revealed that miR-21 expression was reduced in the foci of focal cerebral infarction in mice and N2a cells subjected to OGD.
The first miRNAs identified were members of the let-7 family, which are highly conserved across species.22 Although less is known about let-7’s involvement in ECs, its relevance to the pathophysiology of cancer has been extensively documented.27 According to a study by Xiang et al, let-7 family miRNA expression was downregulated in human brain microvascular endothelial cells (HBMECs) following OGD application. HBMECs were shielded from OGD damage, and inflammation was decreased by let-7i overexpression. The anti-inflammatory effects of let-7i were eliminated when TLR4 expression was silenced.28 Eventually, it was discovered that let-7 family miRNAs were downregulated in an OGD model, after examining the connection between the let-7 family of miRNAs and the in vitro ischemic stroke model. The outcomes demonstrated that let-7i has a protective function for ECs. After thrombolysis, plasma levels of let-7i were elevated, which may help with vascular healing. Further investigation is necessary to clarify the role of let-7i in the in vivo endothelium in ischemic stroke.28
The aging population and the increase in the number of stroke survivors are currently contributing to the rise in the prevalence of post-stroke cognitive impairment (PSCI).29 Wang et al discovered the overexpression of miR-let-7i in PCSI, in line with earlier results.30 They showed that miR-let-7i could target Bcl-2 to protect cells from OGD-induced cell damage in vitro. Additionally, it has also been demonstrated that miR-let-7i can reduce EC loss in the OGD model.30 Several studies have also demonstrated that miRNAs, including let-7, miR-195, miR-122, and miR-203, have anti-apoptotic impacts that protect cells from harm.31-34 Consequently, miRNAs may become a novel therapeutic target for neurological disorders.
Four amino acids are combined to create the synthetic peptide GA, which has a fixed molar residue ratio.15 Real-time PCR analysis in the present study showed that GA decreases miR-21 and let-7i expression in the stroke mice model and the PBMCs of stroke patients. GA has been used clinically for many years to treat relapsing-remitting multiple sclerosis and has been shown to reduce experimental allergic encephalomyelitis.15 Although the precise mechanism of GA in autoimmune neuroinflammation is unknown, it is thought to act by blocking pro-inflammatory Th1 cytokines, activating Th2 cells and Tregs, and reducing monocyte reactivity.16 Another possible mechanism is that GA provides direct neuroprotective effects, for instance, via brain-derived neurotrophic factor.35 The present study found that the GA diminished the expression of microRNAs by downregulating the MAPK and JAK pathways in stroke mice models. Dreikorn et al conducted a meta-analysis of four trials that examined the use of GA in experimental stroke out of all the immunotherapeutic methods approved for multiple sclerosis.36 They found that GA prevented axonal and neuronal degeneration; however, the drug had no effects in animal models of amyotrophic lateral sclerosis.23 It is worth noting that this study has some limitations, including the sample size and lack of proper follow-up, which should be addressed in future studies.
Conclusion
Although GA may partially preserve neurons in neurodegenerative diseases, its use in ischemic stroke seems less promising. The present study concluded that GA, a random polymer composed of four amino acids found in myelin basic protein, produces anti-inflammatory cytokines and can decrease miR-21 and let-7i expression by downregulating MAPK and JAK in stroke models. Further studies in animal models and human patients are needed to investigate its therapeutic effect.
Authors’ Contribution
Conceptualization: Milad Golizadeh, Nima Sadeghi.
Data curation: Nima Sadeghi.
Formal analysis: Milad Golizadeh.
Investigation: Nima Sadeghi.
Methodology: Milad Golizadeh Nima Sadeghi.
Project administration: Paria Kahnamouyi.
Resources: Nima Sadeghi.
Software: Milad Golizadeh.
Supervision: Paria Kahnamouyi.
Validation: Paria Kahnamouyi.
Visualization: Nima Sadeghi.
Writing–original draft: Milad Golizadeh.
Writing–review & editing: Reza Salehi.
Competing Interests
The authors declare no competing interests.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
This study was approved by Research Ethics Committees of Tabriz University of Medical Sciences (ethical code: Research Ethics Committees of Tabriz University of Medical Sciences).
Acknowledgements
The present study was conducted at the Neuroscience Research Center and the Immunology Research Center of Tabriz University of Medical Sciences.
References
- Dirnagl U, Endres M. Found in translation: preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 2014; 45(5):1510-8. doi: 10.1161/strokeaha.113.004075 [Crossref] [ Google Scholar]
- Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta 2016; 1862(3):461-71. doi: 10.1016/j.bbadis.2015.10.018 [Crossref] [ Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42(9):2672-713. doi: 10.1161/STR.0b013e3182299496 [Crossref] [ Google Scholar]
- Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372(24):2296-306. doi: 10.1056/NEJMoa1503780 [Crossref] [ Google Scholar]
- Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C. Reperfusion therapy in acute ischemic stroke: dawn of a new era?. BMC Neurol 2018; 18(1):8. doi: 10.1186/s12883-017-1007-y [Crossref] [ Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009; 40(5):1849-57. doi: 10.1161/strokeaha.108.534503 [Crossref] [ Google Scholar]
- Poittevin M, Deroide N, Azibani F, Delcayre C, Giannesini C, Levy BI. Glatiramer Acetate administration does not reduce damage after cerebral ischemia in mice. J Neuroimmunol 2013; 254(1-2):55-62. doi: 10.1016/j.jneuroim.2012.09.009 [Crossref] [ Google Scholar]
- Love S, Louis D, Ellison DW. Greenfield’s Neuropathology Eighth Edition 2-Volume Set. CRC Press; 2008.
- Grønberg NV, Johansen FF, Kristiansen U, Hasseldam H. Leukocyte infiltration in experimental stroke. J Neuroinflammation 2013; 10:115. doi: 10.1186/1742-2094-10-115 [Crossref] [ Google Scholar]
- Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci 2017; 18(10):2135. doi: 10.3390/ijms18102135 [Crossref] [ Google Scholar]
- Lehmann J, Härtig W, Seidel A, Füldner C, Hobohm C, Grosche J. Inflammatory cell recruitment after experimental thromboembolic stroke in rats. Neuroscience 2014; 279:139-54. doi: 10.1016/j.neuroscience.2014.08.023 [Crossref] [ Google Scholar]
- Kraft P, Göbel K, Meuth SG, Kleinschnitz C. Glatiramer acetate does not protect from acute ischemic stroke in mice. Exp Transl Stroke Med 2014; 6(1):4. doi: 10.1186/2040-7378-6-4 [Crossref] [ Google Scholar]
- Tselis A, Khan O, Lisak RP. Glatiramer acetate in the treatment of multiple sclerosis. Neuropsychiatr Dis Treat 2007; 3(2):259-67. doi: 10.2147/nedt.2007.3.2.259 [Crossref] [ Google Scholar]
- Blanco Y, Moral EA, Costa M, Gómez-Choco M, Torres-Peraza JF, Alonso-Magdalena L. Effect of glatiramer acetate (Copaxone®) on the immunophenotypic and cytokine profile and BDNF production in multiple sclerosis: a longitudinal study. Neurosci Lett 2006; 406(3):270-5. doi: 10.1016/j.neulet.2006.07.043 [Crossref] [ Google Scholar]
- Dhib-Jalbut S. Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis. Neurology 2002; 58(8 Suppl 4):S3-9. doi: 10.1212/wnl.58.8_suppl_4.s3 [Crossref] [ Google Scholar]
- Lalive PH, Neuhaus O, Benkhoucha M, Burger D, Hohlfeld R, Zamvil SS. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs 2011; 25(5):401-14. doi: 10.2165/11588120-000000000-00000 [Crossref] [ Google Scholar]
- Aharoni R. Immunomodulation neuroprotection and remyelination - the fundamental therapeutic effects of glatiramer acetate: a critical review. J Autoimmun 2014; 54:81-92. doi: 10.1016/j.jaut.2014.05.005 [Crossref] [ Google Scholar]
- Weber MS, Starck M, Wagenpfeil S, Meinl E, Hohlfeld R, Farina C. Multiple sclerosis: glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain 2004; 127(Pt 6):1370-8. doi: 10.1093/brain/awh163 [Crossref] [ Google Scholar]
- Cruz Y, García EE, Gálvez JV, Arias-Santiago SV, Carvajal HG, Silva-García R. Release of interleukin-10 and neurotrophic factors in the choroid plexus: possible inductors of neurogenesis following copolymer-1 immunization after cerebral ischemia. Neural Regen Res 2018; 13(10):1743-52. doi: 10.4103/1673-5374.238615 [Crossref] [ Google Scholar]
- Cruz Y, Lorea J, Mestre H, Kim-Lee JH, Herrera J, Mellado R. Copolymer-1 promotes neurogenesis and improves functional recovery after acute ischemic stroke in rats. PLoS One 2015; 10(3):e0121854. doi: 10.1371/journal.pone.0121854 [Crossref] [ Google Scholar]
- Poittevin M, Deroide N, Azibani F, Delcayre C, Giannesini C, Levy BI. Glatiramer acetate administration does not reduce damage after cerebral ischemia in mice. J Neuroimmunol 2013; 254(1-2):55-62. doi: 10.1016/j.jneuroim.2012.09.009 [Crossref] [ Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116(2):281-97. doi: 10.1016/s0092-8674(04)00045-5 [Crossref] [ Google Scholar]
- Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics 2011; 43(10):521-8. doi: 10.1152/physiolgenomics.00158.2010 [Crossref] [ Google Scholar]
- Li X, Wei Y, Wang Z. microRNA-21 and hypertension. Hypertens Res 2018; 41(9):649-61. doi: 10.1038/s41440-018-0071-z [Crossref] [ Google Scholar]
- Zhou J, Zhang J. Identification of miRNA-21 and miRNA-24 in plasma as potential early stage markers of acute cerebral infarction. Mol Med Rep 2014; 10(2):971-6. doi: 10.3892/mmr.2014.2245 [Crossref] [ Google Scholar]
- Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A. MicroRNA-21 protects neurons from ischemic death. FEBS J 2010; 277(20):4299-307. doi: 10.1111/j.1742-4658.2010.07818.x [Crossref] [ Google Scholar]
- Bao MH, Feng X, Zhang YW, Lou XY, Cheng Y, Zhou HH. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int J Mol Sci 2013; 14(11):23086-102. doi: 10.3390/ijms141123086 [Crossref] [ Google Scholar]
- Xiang W, Tian C, Peng S, Zhou L, Pan S, Deng Z. Let-7i attenuates human brain microvascular endothelial cell damage in oxygen glucose deprivation model by decreasing toll-like receptor 4 expression. Biochem Biophys Res Commun 2017; 493(1):788-93. doi: 10.1016/j.bbrc.2017.08.093 [Crossref] [ Google Scholar]
- Jacquin A, Binquet C, Rouaud O, Graule-Petot A, Daubail B, Osseby GV. Post-stroke cognitive impairment: high prevalence and determining factors in a cohort of mild stroke. J Alzheimers Dis 2014; 40(4):1029-38. doi: 10.3233/jad-131580 [Crossref] [ Google Scholar]
- Wang ZQ, Li K, Huang J, Huo TT, Lv PY. MicroRNA Let-7i Is a promising serum biomarker for post-stroke cognitive impairment and alleviated OGD-induced cell damage in vitro by regulating Bcl-2. Front Neurosci 2020; 14:215. doi: 10.3389/fnins.2020.00215 [Crossref] [ Google Scholar]
- Han L, Zhou Y, Zhang R, Wu K, Lu Y, Li Y. MicroRNA Let-7f-5p promotes bone marrow mesenchymal stem cells survival by targeting caspase-3 in Alzheimer disease model. Front Neurosci 2018; 12:333. doi: 10.3389/fnins.2018.00333 [Crossref] [ Google Scholar]
- Cheng HY, Wang YS, Hsu PY, Chen CY, Liao YC, Juo SH. miR-195 has a potential to treat ischemic and hemorrhagic stroke through neurovascular protection and neurogenesis. Mol Ther Methods Clin Dev 2019; 13:121-32. doi: 10.1016/j.omtm.2018.11.011 [Crossref] [ Google Scholar]
- Yang Z, Zhong L, Zhong S, Xian R, Yuan B. miR-203 protects microglia mediated brain injury by regulating inflammatory responses via feedback to MyD88 in ischemia. Mol Immunol 2015; 65(2):293-301. doi: 10.1016/j.molimm.2015.01.019 [Crossref] [ Google Scholar]
- Guo D, Ma J, Li T, Yan L. Up-regulation of miR-122 protects against neuronal cell death in ischemic stroke through the heat shock protein 70-dependent NF-κB pathway by targeting FOXO3. Exp Cell Res 2018; 369(1):34-42. doi: 10.1016/j.yexcr.2018.04.027 [Crossref] [ Google Scholar]
- Kala M, Miravalle A, Vollmer T. Recent insights into the mechanism of action of glatiramer acetate. J Neuroimmunol 2011; 235(1-2):9-17. doi: 10.1016/j.jneuroim.2011.01.009 [Crossref] [ Google Scholar]
- Dreikorn M, Milacic Z, Pavlovic V, Meuth SG, Kleinschnitz C, Kraft P. Immunotherapy of experimental and human stroke with agents approved for multiple sclerosis: a systematic review. Ther Adv Neurol Disord 2018; 11:1756286418770626. doi: 10.1177/1756286418770626 [Crossref] [ Google Scholar]