Biomed Res Bull. 2(4):162-171.
doi: 10.34172/biomedrb.2024.22
Original Article
Investigating the Therapeutic Effects of Melissa Officinalis on Clinical and Electro Diagnostic Findings of Patients with Type 2 Diabetes
Neda Dolatkhah 1  , Morteza Rajabi 1, Mohammad Rahbar 1, Kazem Shakouri 1, Yasaman Mirzazadeh 2, Sina Janbaz Alamdary 2, 3, Fariba Eslamian 1, Tannaz Novinbahador 4, 5, Kimia Motlagh Asghari 1, *
, Morteza Rajabi 1, Mohammad Rahbar 1, Kazem Shakouri 1, Yasaman Mirzazadeh 2, Sina Janbaz Alamdary 2, 3, Fariba Eslamian 1, Tannaz Novinbahador 4, 5, Kimia Motlagh Asghari 1, * 
Author information:
1Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
2Faculty of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran
3The Faculty of Medical Sciences of the Islamic Azad University, Tabriz Branch, Tabriz, Iran
4Department of Biology, Faculty of Natural Sciences, University of Tabriz, Tabriz, Iran
5Department of Biochemistry and Clinical Laboratories, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Neurological complications and glycemic control are critical to managing type 2 diabetes (T2D). This study aimed to evaluate the effects of a specific intervention with lemon balm capsules on fasting blood glucose (FBS), hemoglobin A1c (HbA1c), and nerve conduction parameters in patients with T2D.
Methods:
In this randomized controlled trial, 64 patients with T2D were enrolled and randomly assigned to either an experimental group (n=32) or a control group (n=32). After exclusion, 30 participants in each group completed the study. Baseline characteristics, FBS, HbA1c, and various nerve conduction parameters were measured pre- and post-intervention.
Results:
The experimental group demonstrated a significant reduction in FBS levels post-intervention compared to the control group (P=0.02). No significant difference was observed in HbA1c levels (P=0.08). Neurologically, the experimental group had a significant increase in tibial motor nerve action potential amplitude (P=0.001) and higher tibial motor nerve conduction velocity (P=0.03) post-intervention. Significant intra-group changes were also noted in peroneal motor nerve action potential amplitude (P=0.002) and sural sensory nerve potential amplitude (P=0.009). Finally, no significant differences were found in ulnar and median nerve parameters or F-wave latency.
Conclusion:
The intervention with lemon balm led to significant improvements in FBS levels and certain nerve conduction parameters in patients with T2D, indicating potential benefits for metabolic control and nerve function.
Keywords: Type 2 diabetes, Fasting blood glucose, Nerve conduction, Lemon balm, Metabolic control
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The authors declare that they received no funding throughout the study.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by insulin resistance and relative insulin deficiency, resulting in prolonged high blood sugar levels. It constitutes over 90% of diabetes cases and places a substantial economic burden on healthcare systems each year.1-3 The American Diabetes Association states that a diagnosis of T2DM is determined by a fasting plasma glucose level equal to or greater than 126 mg/dL (7.0 mmol/L), a 2-hour plasma glucose level equal to or greater than 200 mg/dL (11.1 mmol/L) during an oral glucose tolerance test, a hemoglobin A1c (HbA1c) level equal to or greater than 6.5% (48 mmol/mol), or a random plasma glucose level equal to or greater than 200 mg/dL (11.1 mmol/L) in the presence of typical diabetes symptoms.4
Elevated blood sugar levels can lead to peripheral nerve damage in diabetes, known as diabetic peripheral neuropathy.5 Studies indicate that this is a major issue for diabetic patients, presenting as polyneuropathy, mononeuropathy, or autonomic neuropathy. Symmetrical sensory polyneuropathy is the most common type and a primary risk factor for diabetic foot ulcers and amputations.6 It often manifests as distal sensory disturbances, such as numbness, tingling, or burning, beginning in the feet and spreading proximally.7 Neuropathic pain, typically occurring in the lower limbs and worsening at night, is also one of its characteristics.8 Diabetic peripheral neuropathy is diagnosed based on specific neuropathic symptoms, detailed neurological examinations, or electrodiagnostic studies.9 Pathophysiologically, diabetic neuropathy results from chronic hyperglycemia, autoimmune mechanisms, and microvascular dysfunction.8 Multifactorial mechanisms, including genetic predisposition and environmental factors such as alcohol and heavy metal exposure, are involved in diabetic neuropathy.10 These processes affect sensory, autonomic, and motor nerves, starting from the distal lower limbs and progressing proximally.9,11 The symptoms of peripheral neuropathy include tingling, numbness, severe nerve pain, and muscle weakness.9,11 Autonomic dysfunction can cause blood pressure irregularities, temperature regulation issues, and gastrointestinal disturbances. Diagnosis often relies on clinical symptoms and neurological examinations, with electrodiagnostic tests such as electromyography (EMG) and nerve conduction studies confirming the diagnosis.11-13 Electromyography assesses muscle response to nerve signals, while nerve conduction studies measure the speed and amplitude of electrical signals in nerves, aiding in diagnosing conditions such as carpal tunnel syndrome.13
Effective management of T2D requires a multifaceted approach, which includes lifestyle interventions, medication, and regular monitoring of clinical and biochemical parameters. Despite advancements in conventional treatments, there remains a significant need to explore additional therapeutic options to control blood sugar levels better and reduce complications, especially diabetic neuropathy.14
Natural products and herbal remedies have attracted increasing interest as complementary therapies because of their potential to enhance glycemic control and reduce diabetes-related complications. Lemon balm (Melissa officinalis), known for its anti-inflammatory, antioxidant, and hypoglycemic properties, emerges as a promising candidate.15-18 Pharmacological studies, both clinical and in vitro studies on animals, have demonstrated the neuroprotective and neurological effects of lemon balm and its main active compound, rosmarinic acid.19-21 However, there is a gap in comprehensive clinical trials specifically evaluating its impact on both clinical outcomes and electrodiagnostic findings in T2DM patients.
The primary active ingredients in lemon balm include rosmarinic acid, caffeic acid, and various flavonoids, which are known for their significant anti-inflammatory and antioxidant activities.22,23 These compounds aid in reducing oxidative stress, a key factor in diabetic complications development.23,24 Lemon balm contains essential oils (e.g., citral) which have been shown to have hypoglycemic effects by improving insulin secretion, enhancing insulin sensitivity, and regulating carbohydrate metabolism.24,25 Research suggests that lemon balm may have a positive impact on neuropathy.23 Lemon balm has significant anti-inflammatory properties that can help reduce nerve inflammation and promote the regeneration of damaged tissues in neuropathy.21 Furthermore, by increasing blood flow and maintaining vascular function, it may help improve nerve function. Its significant antioxidant properties may also be effective in preventing neuronal damage and preserving function.26-28 Some findings indicate that the rosmarinic acid extract may be effective in reducing the degeneration of motor neurons.29 Furthermore, its antioxidant properties help in mitigating oxidative stress, which is implicated in the development of insulin resistance and beta-cell dysfunction. The anti-inflammatory effects of lemon balm may also play a role in improving insulin sensitivity and reducing chronic low-grade inflammation, commonly observed in T2DM.30 Considering that oxidative stress and reactive oxygen species can contribute to the onset of diabetes and its associated complications, consuming natural antioxidants such as lemon balm essential oil may be advantageous in preventing or alleviating diabetes symptoms and complications. Some studies have reported that lemon balm essential oil can lower blood sugar levels because of its antioxidant properties.31
Lemon balm may potentially produce its hypoglycemic effects through multiple pathways at a mechanistic level. It has been discovered that rosmarinic acid can hinder crucial enzymes that are responsible for glucose metabolism, including alpha-amylase and alpha-glucosidase. Consequently, this inhibition leads to a reduction in postprandial hyperglycemia.30 Lemon balm is typically viewed as a safe remedy. Some research has documented certain negative outcomes such as nausea, vomiting, uneasiness, stomach discomfort, and dizziness after using this plant. However, in most instances, these reported side effects demonstrated no significant variation when compared to the control group.32
This study aims to evaluate the therapeutic effects of lemon balm capsules on clinical and electrodiagnostic findings in patients with T2DM. By assessing the impact of lemon balm supplementation on glycemic control, neuropathic symptoms, and nerve conduction velocities, this research seeks to provide a holistic evaluation of its potential benefits. The inclusion of electrodiagnostic assessments offers an objective measure of peripheral nerve function, addressing the prevalent issue of diabetic neuropathy with precise and quantifiable data. Through a rigorous clinical trial design, this study will bridge the existing gap by providing robust evidence on the efficacy of lemon balm capsules as a complementary therapy in diabetes management. The findings can pave the way for integrating lemon balm into clinical practice, potentially enhancing patient outcomes and the quality of life of those living with T2DM.
Methods
Trial Design
This double-blind, randomized clinical trial was conducted at the Endocrinology Clinic of Imam Reza Hospital in Tabriz from March 21, 2021, to March 20, 2023. Participants were provided with a brief explanation of the research objectives and procedures and were enrolled after providing written informed consent if they met the inclusion and exclusion criteria and were willing to participate. Patient information was kept confidential throughout the study. Participants received routine treatments without incurring any costs. This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1399.687) and registered on the Iranian Registry of Clinical Trials website (IRCT20200612047739N1; Registration date: 02.01.2021). The trial followed the CONSORT checklist for clinical trials to ensure comprehensive reporting and adherence to high-quality standards.
Participants
The study focused on patients with T2DM referred from the Tabriz Diabetes Center. Certified endocrinologists assessed all patients to ensure they met the inclusion criteria; they included being 18 years old or older, being diagnosed with T2DM for at least one year, having a body mass index (BMI) between 18.5 and 30, being on a stable dose of antidiabetic medication for at least one month, and exhibiting sensory neuropathy symptoms such as tingling, burning, pain, muscle weakness, atrophy, and balance disorders. On the other hand, the exclusion criteria included type 1 diabetes or other specific types of diabetes, pregnancy, recent insulin therapy (within the past three months), and serious digestive diseases (e.g., peptic ulcer or gastrointestinal bleeding). The other exclusion criteria were autoimmune diseases, thyroid disorders, use of drugs affecting neuropathy symptoms (e.g., antidepressants or antispasmodics), and sensitivity to M. officinalis.
Interventions
Demographic and clinical information was collected and recorded. For cases where neuropathy was not confirmed by the nerve conduction study, the Michigan Neuropathy Screening Instrument (MNSI) was used to assess diabetic neuropathic symptoms. Certified endocrinologists confirmed the neuropathy diagnosis. Upon admission, nerve conduction velocity and movement tests were used to determine the presence of neuropathy.
Participants were randomly divided into either the treatment group receiving M. officinalis or the control group. The treatment group received 1 g of M. officinalis in 500 mg capsules, while the control group received maltodextrin capsules every 12 hours for 3 months. Electrodiagnostic studies using electromyography examined motor nerves (median and tibial) and sensory nerves (sural).
Outcomes
The primary outcome measure was the change in fasting blood glucose (FBG) levels. Secondary outcomes included the assessment of diabetic neuropathic symptoms using the MNSI, nerve conduction velocity, and movement tests. Electrodiagnostic studies were performed to evaluate motor and sensory nerve functions.
Michigan Neuropathy Screening Instrument
The MNSI was utilized to assess diabetic neuropathy symptoms. The first part consisted of 15 questions regarding sensory disturbances. Each question carried a score of 1, with a maximum possible score of 13. The second part included a brief physical examination of the feet, vibration perception threshold testing, Achilles tendon reflex testing, and monofilament testing.
Vibration Test
A 128 Hz tuning fork was placed on the dorsum of the first toe and medial malleolus. With the patient’s eyes closed, they indicated when they could no longer feel the vibration. A difference of more than 10 seconds between the patient’s and examiner’s perception indicated reduced vibration perception.
Semmes-Weinstein Monofilament Test
A 10 g monofilament was applied to designated points on the plantar surface of the foot. The patient, with eyes closed, indicated when they felt the monofilament. Identifying 8 out of 10 points was considered normal; 7 points represented reduced protective sensations, while failure to identify any points demonstrated the absence of protective sensations.
Sample Size
Using G*Power software and the data of a similar study by Asadi et al,30 the primary outcome measure was the change in FBG levels. Considering a 90% power, a type I error of 0.05, and a 15% loss to follow-up, the final sample size was calculated to be 30 individuals per group, totaling 60 participants (30 in the lemon balm treatment group and 30 in the control group).
Randomization and Blinding
Participants were randomly assigned to the treatment or control group using block randomization. Six permutations (AABB, ABAB, ABBA, BAAB, BBAA, and BABA) were used to classify patients, who were then randomly selected for group assignment. In the M. officinalis group, patients received 1 g of M. officinalis in the form of 500 mg capsules, divided into two doses, for three months. The placebo group received identical capsules containing maltodextrin. Both patients and researchers were blinded to the treatment allocation, and the drugs were dispensed in identical packages labeled with unique codes by a third party.
Statistical Analysis
All statistical analyses were performed using the SPSS software (version 23.0, SPSS Inc. in Chicago, IL). Descriptive statistics included frequencies, percentages, means, and standard deviations. The normality of data distribution was assessed using Shapiro-Wilk and Kolmogorov-Smirnov tests, and non-parametric tests were employed when data distribution was not normal. The independent t-test and Mann-Whitney U test were utilized for intergroup comparisons before the intervention, depending on the normality of the data. A P value less than 0.05 was considered statistically significant.
Results
Participant Flow
At the start of the study, 64 patients diagnosed with T2D were enrolled and randomly assigned to either the experimental group (n = 32) or the control group (n = 32) after obtaining consent. During the treatment, 2 individuals from the experimental group and 2 individuals from the control group were excluded due to failure to complete the treatment period. Ultimately, 60 participants were analyzed, including those in the experimental group (n = 30) and the control group (n = 30). The flow of participants is illustrated in Figure 1.
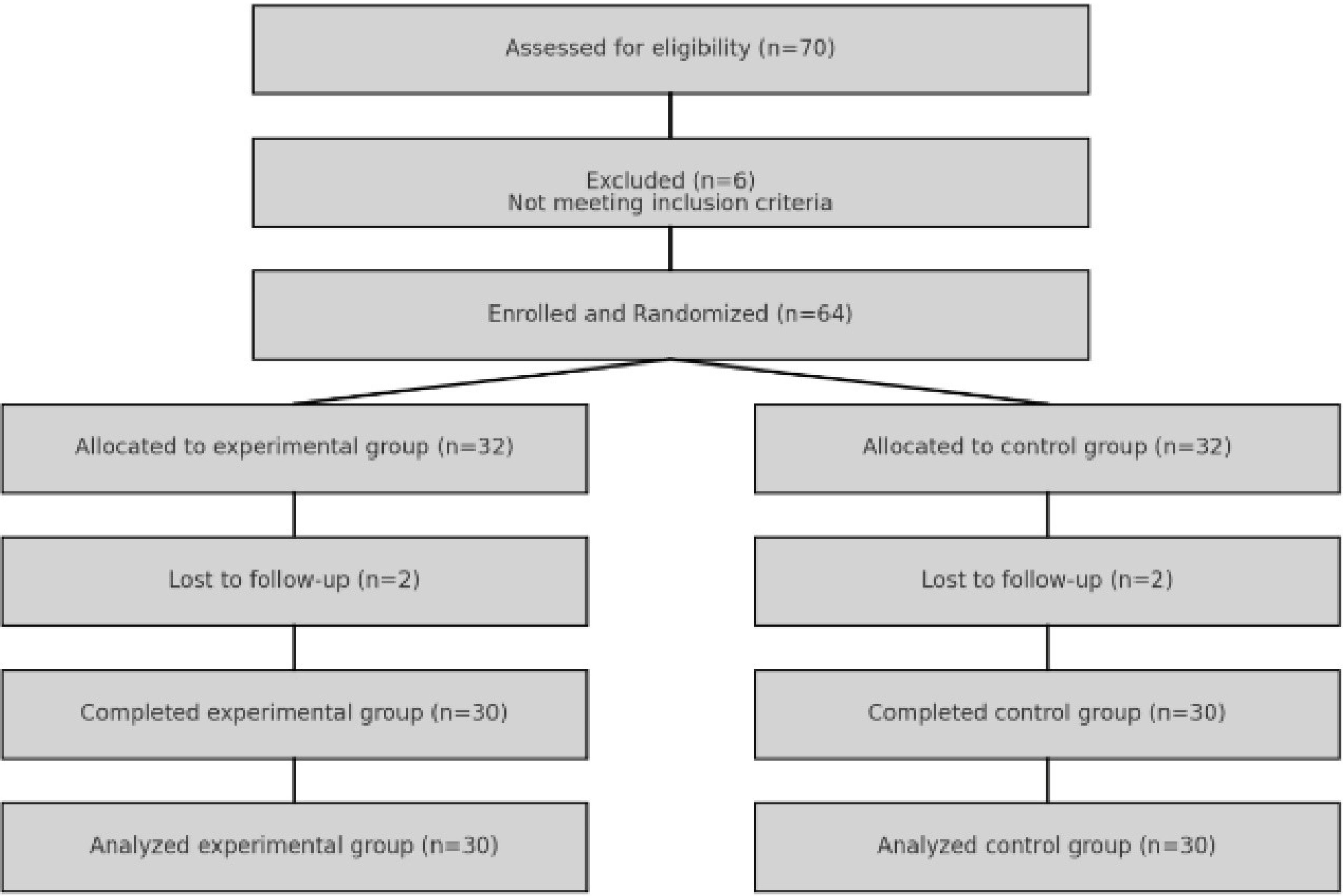
Figure 1.
Flow of Participants
.
Flow of Participants
Baseline Characteristics
The Shapiro-Wilk test was applied to ensure the normality of the data and determine the appropriate statistical test for interval variables. The mean age of patients with T2D was 48.91 ± 7.15 years, with a range of 35–71 years. The majority of patients were 50 years or older (75%). The mean age in the experimental and control groups was 43.9 ± 6.76 (35–70 years) and 49.9 ± 7.43 (39–71 years), respectively. The comparison of mean age between the two groups indicated no significant difference, suggesting that the groups were homogeneous in terms of age (t = 0.54, P = 0.59).
Overall, 38.3% (n = 23) of the participants were male, and 61.7% (n = 37) were female. The comparison of gender frequency between the two groups revealed that 26.7% and 50% of patients in the control and experimental groups were male, respectively. Similarly, 73.3% and 50% of participants in the control and experimental groups were female, respectively. The difference in gender distribution between the two groups was not significant (χ2 = 3.45, P= 0.06), implying that the groups were homogeneous in terms of gender. There were no significant differences between the two groups with regard to other underlying and baseline variables, FBS (Z = 1.52, P = 0.21), and HbA1c (Z = 0.13, P = 0.71). These variables were homogeneously distributed between the two groups (P > 0.05). The characteristics of participants are detailed in Table 1.
Table 1.
Baseline Demographic and Clinical Characteristics of the Participants in the Two Study Groups at Baseline
|
Variable
|
Intervention Group
|
Control Group
|
P
Value
|
|
(n=30)
|
(n=30)
|
| Gender, n (%) |
|
|
0.06* |
| Female |
22 (73.3%) |
15 (50%) |
|
| Male |
8 (26.7%) |
15 (50%) |
|
| Age (y) |
43.9 ± 6.76 |
49.9 ± 7.43 |
0.59** |
| Education, n (%) |
|
|
0.561*** |
| Elementary |
12 (40%) |
15 (50%) |
|
| Diploma |
12 (40%) |
11 (36.7%) |
|
| College |
6 (20%) |
3 (13.3%) |
|
| Job |
|
|
0.707*** |
| Employed |
8 (26.7%) |
8 (26.7%) |
|
| Unemployed |
7 (23.3%) |
5 (18.3%) |
|
| Retired |
4 (13.3%) |
5 (18.3%) |
|
| Housewife |
11 (36.7%) |
11 (36.7%) |
|
| Specific diet |
|
|
0.001 |
| Yes |
0 (0%) |
0 (0%) |
|
| No |
32 (100%) |
31 (100%) |
|
| Family history of diabetes |
|
|
0.124*** |
| Yes |
18 (60%) |
14 (46.6%) |
|
| No |
12 (40%) |
16 (53.4%) |
|
| Duration of diabetes |
|
|
0.328*** |
| Less than 5 years |
19 (63.3%) |
15 (50%) |
|
| 5 years and more |
11 (36.7%) |
15 (50%) |
|
| Anti-glycemic drugs |
|
|
0.741*** |
| Biguanides |
0 (0%) |
2 (3.3%) |
|
| Sulfonylureas |
7 (23.3%) |
8 (26.7%) |
|
| Thiazolidinediones |
4 (13.4%) |
5 (18.3%) |
|
| Combination blood glucose-lowering drugs (oral) |
|
|
0.001 |
| Insulins/analogs long-acting |
12 (40%) |
10 (33.4%) |
|
| + Insulins/analogs Intermediate-acting |
7 (23.3%) |
5 (18.3 %) |
|
| Weight (kg) |
80.4 ± 7.55 |
76.6 ± 8.21 |
0.108* |
| Body mass index (kg/m2), mean ± SD |
29.8 ± 5.44 |
27.3 ± 4.25 |
0.329* |
| FBS before intervention (Mg/DL), mean ± SD |
213.46 ± 53.37 |
235.3 ± 52.60 |
0.21** |
| Hemoglobin A1c (Mg/Dl), mean ± SD |
8.39 ± 2.01 |
9.12 ± 1.32 |
0.71** |
Note. FBS: Fasting blood glucose; SD: Standard deviation. All values are presented as means ± SD or No. (%).
*Chi-square goodness of fit test
**T-test
**Mann–Whitney U test
***Chi-square test of independence
Primary Outcome
Based on the results (Table 2), there was no significant difference between the groups regarding the baseline variables FBS and HbA1c (P > 0.05). After intervention, the mean FBS in the experimental group was significantly lower than that in the control group (Z = -2.26, P= 0.02). The comparison of mean HbA1c after the intervention showed no significant difference between the two groups (Z = -1.98, P= 0.08).
Table 2.
Comparison of Blood Sugar and Hemoglobin A1c Variables in Patients With Diabetes Before and After the Intervention
| Variable |
|
Intervention Group
|
Control Group
|
P
Value
|
| FBS before intervention (mg/dL), mean ± SD |
Before intervention |
213.46 ± 53.37 |
235.3 ± 60.52 |
0.21 |
| After intervention |
235.26 ± 61.05 |
196.33 ± 52.38 |
0.01 |
| Hemoglobin A1c (mg/dL), mean ± SD |
Before intervention |
8.93 ± 2.01 |
9.21 ± 1.32 |
0.71 |
| After intervention |
9.23 ± 1.23 |
8.72 ± 1.205 |
0.08 |
Note. FBS: Fasting blood glucose; SD: Standard deviation.
Secondary Outcomes
The mean changes in tibial motor nerve action potential amplitude and tibial motor nerve conduction velocity in both groups before and after the intervention are presented in Table 3.
Table 3.
Comparison of the Tibial Nerve Mean Conduction Velocity and Action Potential Amplitude, Between the Two Groups
| Variable |
|
Intervention Group
|
Control Group
|
P
Value
|
| Amplitude of the tibial motor nerve potential |
Before intervention |
1.47 ± 1.85 |
1.45 ± 1.43 |
0.92a |
| After intervention |
2.14 ± 1.92 |
1.37 ± 1.24 |
0.12a |
| Intergroup changes |
0.4 ± 1.33 |
0.08 ± 0.406 |
0.001a |
| Tibial motor nerve conduction velocity |
Before intervention |
38.96 ± 4.15 |
36.86 ± 3.308 |
0.03b |
| After intervention |
38.13 ± 3.54 |
36.36 ± 1.84 |
0.03a |
| Intergroup changes |
0.83 ± 3.58 |
0.5 ± 2.78 |
0.75a |
aMann–Whitney U test.
bt test.
Additionally, intra-group changes were compared, and no significant difference was found between the control and intervention groups in terms of tibial motor nerve action potential amplitude before the intervention (Z = -0.09, P = 0.92). Similarly, there was no significant difference between the two groups regarding tibial motor nerve action potential amplitude after the intervention (Z = -1.51, P= 0.12). Nonetheless, the analysis of intra-group changes in tibial motor nerve action potential amplitude revealed a significant difference, with greater changes in the intervention group compared to the control group (Z = -3.38, P= 0.001). The two groups had a significant difference in terms of tibial motor nerve conduction velocity before the intervention, with higher velocity in the intervention group compared to the control group (t = -2.16, P= 0.03). Post-intervention evaluation also demonstrated higher velocity in the intervention group (Z = -2.09, P= 0.03). However, considering the insignificance of intra-group changes, the results suggested no effect of the intervention on tibial motor nerve conduction velocity (Z = -0.31, P= 0.75).
Table 4 presents the comparison results between the mean changes in motor nerve action potential amplitude and conduction velocity of the peroneal motor nerve in the two groups before and after the intervention, as well as intra-group changes. Based on the findings, there was no significant difference between the control and intervention groups in terms of the motor nerve action potential amplitude (Z = -0.14, P= 0.88) and peroneal motor nerve conduction velocity (Z = -1.32, P = 0.18) before the intervention. Likewise, no significant difference was observed between the control and intervention groups regarding motor nerve action potential amplitude (Z = -1.59, P= 0.11) and peroneal motor nerve conduction velocity (Z = -0.75, P = 0.45) after the intervention. Intra-group change analysis confirmed a significant difference between the two groups in terms of motor nerve action potential amplitude (Z = -3.08, P= 0.002). However, intra-group changes in peroneal motor nerve conduction velocity were not significant between the two groups (Z = -0.64, P = 0.51), the details of which are provided in Table 4.
Table 4.
Comparison of the Peroneal Motor Nerve Mean Conduction Velocity and Peroneal Motor Nerve Action Potential Amplitude Between the Two Groups
| Variable |
|
Intervention Group
|
Control Group
|
P
Valuea
|
| Amplitude of the peroneal motor nerve potential |
Before intervention |
0.83 ± 0.81 |
0.8 ± 0.902 |
0.88 |
| After intervention |
1.06 ± 1.07 |
0.65 ± 0.73 |
0.11 |
| Intergroup changes |
0.230 ± 0.53 |
0.15 ± 0.43 |
0.002 |
| Peroneal motor nerve conduction velocity |
Before intervention |
39.21 ± 0.84 |
38.23 ± 2.95 |
0.18 |
| After intervention |
37.73 ± 3.53 |
37.5 ± 2.67 |
0.45 |
| Intergroup changes |
1.36 ± 2.52 |
0.73 ± 2.34 |
0.51 |
Table 5 summarizes the comparison between the mean changes in ulnar motor nerve action potential amplitude and ulnar sensory nerve action potential amplitude in both groups before and after the intervention, as well as intra-group changes. The findings showed no significant difference between the two groups with regard to ulnar motor nerve action potential amplitude (Z = -0.73, P= 0.46) and ulnar sensory nerve action potential amplitude (Z = -0.401, P= 0.68). After the intervention, there was also no significant difference between the control and intervention groups regarding ulnar motor nerve action potential amplitude (Z = -1.68, P= 0.09) and ulnar sensory nerve action potential amplitude (Z = -1.58, P = 0.11). Based on the analysis of intra-group changes, no significant difference was detected between the two groups in terms of ulnar motor nerve action potential amplitude (Z = -0.26, P = 0.78). However, intra-group changes in ulnar sensory nerve action potential amplitude were significant between the two groups (Z = -1.98, P = 0.04).
Table 5.
Comparison of the Mean Amplitude of the Ulnar Sensory and Motor Nerve Potential Before and After the Intervention in the Two Groups
| Variable |
|
Intervention Group
|
Control Group
|
P
Valuea
|
| Amplitude of the ulnar motor nerve potential |
Before intervention |
5.75 ± 2.15 |
5.34 ± 1.56 |
0.88 |
| After intervention |
6.51 ± 1.89 |
5.903 ± 1.94 |
0.11 |
| Intergroup changes |
-0.76 ± 1.35 |
-0.56 ± 1.03 |
0.002 |
| Amplitude of the ulnar sensory nerve potential |
Before intervention |
11.78 ± 5.78 |
12.08 ± 4.44 |
0.18 |
| After intervention |
11.13 ± 5.29 |
14.1 ± 9.506 |
0.45 |
| Intergroup changes |
0.65 ± 2.36 |
-2.01 ± 7.33 |
0.51 |
Table 6 lists the mean variables of median motor nerve conduction velocity, median motor nerve action potential amplitude, and median sensory nerve action potential amplitude in both groups before and after the intervention, as well as intra-group changes. The comparison of the mean variables of median motor nerve conduction velocity (Z = -0.89, P = 0.42), median motor nerve action potential amplitude (Z = -1.08, P= 0.27), and median sensory nerve action potential amplitude (Z = -0.55, P = 0.57) before the intervention revealed no significant difference between the two groups. After the intervention, there was still no significant difference between the two groups regarding median motor nerve conduction velocity (Z = -1.05, P = 0.22), median motor nerve action potential amplitude (Z = -0.21, P = 0.83), and median sensory nerve action potential amplitude (Z = -0.43, P = 0.15). The analysis of intra-group changes also demonstrated no significant difference between the two groups in terms of the variables of median motor nerve conduction velocity (Z < 0.01, P > 0.99), median motor nerve action potential amplitude (Z = -0.89, P = 0.37), and median sensory nerve action potential amplitude (Z = -0.98, P = 0.32).
Table 6.
Comparison of the Median Nerve Mean Conduction Velocity, Motor Nerve Action Potential Amplitude, and Median Sensory Nerve Action Potential Amplitude Between the Two Groups
| Variable |
|
Intervention Group
|
Control Group
|
P
Valuea
|
| Median motor nerve conduction velocity |
Before intervention |
10.87 ± 6.32 |
11.9 ± 4.49 |
0.42 |
| After intervention |
10.37 ± 4.72 |
13.05 ± 11.04 |
0.22 |
| Intergroup changes |
0.5 ± 4.23 |
1.15 ± 8.95 |
> 0.99 |
| Amplitude of the median motor nerve potential |
Before intervention |
5.63 ± 2.21 |
5.76 ± 2.05 |
0.27 |
| After intervention |
5.91 ± 1.94 |
5.96 ± 2.35 |
0.83 |
| Intergroup changes |
0.55 ± 2.29 |
0.19 ± 1.34 |
0.37 |
| Amplitude of the median sensory nerve potential |
Before intervention |
48.66 ± 4.23 |
48.2 ± 4.75 |
0.57 |
| After intervention |
48.53 ± 3.32 |
47.4 ± 3.43 |
0.15 |
| Intergroup changes |
0.13 ± 4.28 |
0.8 ± 5.33 |
0.32 |
The mean amplitude of the sural sensory nerve potential was compared between the two groups before and after the intervention, as well as the changes within the groups (Table 7). The comparison of the mean amplitude of the sural sensory nerve potential before the intervention showed no significant difference between the two groups (Z = -0.26, P = 0.78). However, after the intervention, the two groups had a significant difference in terms of the sural sensory nerve potential amplitude (Z = -6.86, P = 0.009). The intra-group changes analysis also represented a significant difference between the two groups with regard to the sural sensory nerve potential amplitude (Z = -2.55, P = 0.01).
Table 7.
Comparison of the Mean Amplitude of the Sural Sensory Nerve Potential Before and After the Intervention in the Two Groups
|
Variables
|
|
Intervention Group
|
Control Group
|
P
Valuea
|
| Amplitude of the sural sensory nerve potential |
Before intervention |
2.86 ± 3.207 |
5.24 ± 3.71 |
0.78 |
| After intervention |
4.6 ± 4.57 |
5.03 ± 3.89 |
0.009 |
| Intergroup changes |
0.206 ± 2.16 |
1.73 ± 3.18 |
0.01 |
Table 8 provides the mean of the minimum F-wave latency in both groups before and after the intervention. The comparison of the mean minimum F-wave latency before the intervention revealed no significant difference between the two groups (Z = -0.08, P = 0.92). After the intervention, there was no significant difference between the two groups in terms of the mean minimum F-wave latency (Z = -0.62, P = 0.53). The intra-group changes analysis demonstrated no significant difference between the two groups in terms of the mean minimum F-wave latency (Z = -0.46, P = 0.64).
Table 8.
Comparison of the Average Minimum Peroneal F Wave Delay Before and After the Intervention in the Two Groups
|
Variables
|
|
Intervention Group
|
Control Group
|
P
Valuea
|
| Average minimum peroneal F-wave (ms) |
Before intervention |
59.8 ± 3.79 |
59.903 ± 4.65 |
0.92 |
| After intervention |
60.46 ± 3.89 |
55.60 ± 3.74 |
0.53 |
| Intergroup changes |
|
|
0.64 |
Discussion
Based on the findings of the present study, although blood sugar levels decreased after three months of consuming lemon balm, no significant difference was found in HbA1c levels between the two groups. The assessment of electrodiagnostic factors after consuming lemon balm indicated that there was no difference in electrodiagnostic indices between the treatment and control groups. Although the amplitude of the sural nerve sensory action potential was higher in the treatment group compared to the control group, given the presence of neuropathy in the studied patients, reliable recording of sensory nerve action potentials, particularly the sural nerve, can be challenging. Physicians must take into account the presence of various measuring devices and different techniques used in diverse evaluations. Additionally, they should consider the sensitivity of the sensory action potential parameter to environmental conditions and temperature, the level of skin contact, and the cleanliness of the recording and stimulation site. Due to the high variability of this parameter under different conditions, it is challenging to make comprehensive comments on the improvement of sensory action potential.
There is no prior study demonstrating the effect of lemon balm on improving sensory nerve action potential amplitude. However, the results of the present study confirmed that the amplitude of the sural sensory nerve potential was significantly higher in the treatment group compared to the control group. Other electrodiagnostic evaluation factors did not show significant differences between the lemon balm and control groups. To the best of our knowledge, the present study is the first to assess the impact of lemon balm on the neurological functions of diabetic patients. There are no previous studies in scientific literature to either support or contradict these findings.
According to our knowledge, there have been very few studies on the effect of lemon balm on blood sugar control in humans. For instance, Asadi et al30 evaluated the antidiabetic properties of the hydroalcoholic extract of lemon balm in type 2 diabetic patients. The study compared the effects of taking a daily 700 mg lemon balm capsule with a placebo. The results revealed significant differences in fasting blood sugar, HbA1c, pancreatic beta-cell activity, triglycerides, blood lipids, and systolic blood pressure between the two groups. However, there were no significant changes in total cholesterol, low-density lipoprotein, and insulin levels. No adverse effects were observed from consuming lemon balm in the study.31 Our study’s findings align with the observed effect of lemon balm on fasting blood sugar levels. However, this study did not assess the impact of this factor on the lipid profile of patients. In the present study, 500 mg capsules were used, and it appears that doses lower than 700 mg can also effectively reduce blood sugar levels. Other in vitro and in vivo studies have shown significant blood sugar and lipid-lowering effects of lemon balm.26,33,34 Some studies have also reported the beneficial effects of alcoholic extracts and essential oils of lemon balm on blood lipids and liver enzymes.35,36
The usage of herbal remedies can have a substantial impact on the functioning of the nervous and muscular systems. These effects can manifest directly or indirectly through alterations in the electrical activity of nerve and muscle cells. Consequently, there has been an increasing focus on investigating the influence of medications on nerve conduction velocity and electromyographic activity. The impact of medications on nerve conduction velocity and electromyographic activity can be either direct or indirect and can carry significant implications for the functioning of the nervous and muscular systems. Further research is necessary to gain a comprehensive understanding of the mechanisms through which medication affects these physiological processes and to formulate strategies for optimizing medical therapies to minimize their necessity. In this context, lemon balm is among the herbal medicines with therapeutic and antioxidant properties mentioned in some studies. Due to the general inclination of society toward the use of herbal medicines and their fewer side effects compared to other kinds of medications,37-39 the effect of lemon balm extract on the symptoms of diabetic patients was investigated in the present study, possibly due to the presence of flavonoids and antioxidants in this plant. Changes in the liver and adipose tissue were found to be associated with reduced blood glucose and triglyceride levels, without causing any liver damage. Recent studies have observed an increase in the activity and gene expression of a key liver enzyme responsible for glucose regulation, called glucokinase, along with a decrease in the production of important enzymes involved in gluconeogenesis, such as glucose-6-phosphatase and phosphoenolpyruvate carboxykinase. These findings imply the effectiveness of this enzyme in treating diabetes.37
Some studies have confirmed the positive neurological effects of lemon balm. A therapeutic dose for the treatment of certain diseases is believed to be 26 g of dried leaves of the plant and 6 g of dried seeds. This plant is often used in combination with other herbs for the nervous system and the brain.40 Numerous pharmacological and clinical studies have verified the neurological effects and potent neuroprotective properties of different lemon balm extracts and their essential oils in treating various diseases. These conditions include memory impairment, cognitive disorders, sleep disorders, and epilepsy. The plant achieves its effects through various mechanisms, such as inhibiting oxidative stress, acetylcholinesterase inhibition, stimulation of acetylcholine and gamma-aminobutyric acid type-A receptors, and inhibition of metalloproteinases 2 and monoamine oxidase.41-43 The aqueous and methanolic extracts of lemon balm have been shown to significantly reduce the production of free oxygen radicals, indicating their neuroprotective effects.19 According to the findings of a previous study, the aqueous extract of lemon balm demonstrated neuroprotective effects against damage caused by 3,4-methylenedioxy methamphetamine in the primary neuronal cultures of the hippocampal region. This protective effect could be attributed to the antioxidant effects and the inhibition of monoamine oxidase activity. The pre- and post-treatment of human neuronal cells with rosmarinic acid preserves neuronal integrity and reduces nerve damage caused by ciguatoxin toxicity. Therefore, lemon balm essential oil induces neuroprotective effects against cellular death resulting from hypoxia.44 Gallic acid, another key compound found in lemon balm, is capable of inhibiting the enzyme metalloproteinase 2. Some studies have indicated that this enzyme plays a role in the pathology of Alzheimer’s disease.45
The findings of this study have important implications for T2D management, particularly regarding the potential benefits of M. officinalis (lemon balm) on glycemic control and diabetic neuropathy. The significant reduction in FBS levels in the experimental group underscores the potential of lemon balm to enhance immediate glycemic control, which is critical for preventing both acute and chronic complications of diabetes. This suggests that incorporating M. officinalis into routine diabetes management could provide an effective adjunct to traditional therapies, helping patients achieve better blood glucose regulation. Moreover, the observed improvements in tibial motor nerve action potential amplitude and conduction velocity indicate that lemon balm may offer neuroprotective benefits. Given that diabetic neuropathy is a common and debilitating complication of diabetes, the ability to improve nerve function could significantly enhance patients’ quality of life by reducing symptoms such as pain, tingling, and loss of sensation.6 This could also decrease the risk of foot ulcers and subsequent amputations, which are serious complications of diabetic neuropathy.8 The significant intra-group changes in peroneal motor nerve action potential amplitude and sural sensory nerve potential amplitude further support the potential benefits of lemon balm for peripheral nerve health. Improvements in these parameters could translate to better motor control and sensory function in the lower limbs and enhance mobility and overall functionality in patients with T2D.
Limitations of the Study
Despite the promising results, this study had several limitations that need to be addressed in future research.The relatively short follow-up period may not capture the long-term effects of M. officinalis, particularly on HbA1c levels. HbA1c is a measure of average blood glucose during 2–3 months, and changes in this parameter might require a longer duration to become evident. Thus, future studies should consider extending the follow-up period to better understand the sustained impact of lemon balm on glycemic control.
Another limitation is the modest sample size, which, although sufficient to detect significant differences in some parameters, may limit the generalizability of the results. Accordingly, larger studies with more diverse populations are necessary to confirm these findings and ensure they are applicable to a broader range of patients with T2D.Additionally, the study did not find significant changes in upper limb nerve parameters (ulnar and median nerve) or F-wave latency, suggesting that the effects of M. officinalis might be more localized to the lower limbs. This raises questions about the overall scope of the intervention’s efficacy and whether it could be adapted or supplemented to achieve more comprehensive neurological benefits.
Eventually, while the study controlled for several baseline characteristics, other potential confounding factors, such as lifestyle habits, adherence to medication, and concurrent therapies, were not thoroughly examined. These factors could influence the outcomes and thus should be considered in future research to isolate the intervention’s true effects.
Conclusion
In summary, this study provides evidence that M. officinalis (lemon balm) can significantly improve FBS levels and certain peripheral nerve conduction parameters in patients with T2D, indicating potential benefits for both metabolic control and neurological health. These findings highlight that lemon balm could be a valuable addition to diabetes management strategies. However, further research is necessary to explore the long-term effects, optimize the intervention parameters, and confirm the results in larger and more diverse populations. Addressing the above limitations will enhance the robustness and applicability of future studies, ultimately contributing to better outcomes for patients with T2D.
Authors’ Contribution
Conceptualization: Neda Dolatkhah,Morteza Rajabi,Kazem Shakouri.
Data curation: Morteza Rajabi,Mohammad Rahbar,Fariba Eslamian.
Formal analysis: Yasaman Mirzazadeh, Sina Janbaz Alamdary, Tannaz Novinbahador.
Investigation: Neda Dolatkhah,Kazem Shakouri.
Methodology: Morteza Rajabi,Mohammad Rahbar.
Project administration: Neda Dolatkhah.
Resources: Morteza Rajabi.
Software: Morteza Rajabi.
Supervision: Kimia Motlagh Asghari,Neda Dolatkhah.
Validation: Morteza Rajabi.
Visualization: Morteza Rajabi.
Writing–original draft: Yasaman Mirzazadeh, Sina Janbaz Alamdary, Tannaz Novinbahador.
Writing–review & editing: Kimia Motlagh Asghari.
Competing Interests
The authors declare that they have no conflict of interests.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request. All relevant data generated and analyzed during the study, including clinical measurements, para-clinical assessments, and statistical analyses, will be made available to qualified researchers who wish to replicate or verify the results presented in the published manuscript.
Ethical Approval
This study was funded by Research Ethics Committees of Islamic Azad University-Tabriz Branch (Code: IR.IAU.TABRIZ.REC.1400.027).
References
- Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet 2017; 389(10085):2239-51. doi: 10.1016/s0140-6736(17)30058-2 [Crossref] [ Google Scholar]
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87(1):4-14. doi: 10.1016/j.diabres.2009.10.007 [Crossref] [ Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27(5):1047-53. doi: 10.2337/diacare.27.5.1047 [Crossref] [ Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes 2022; 40(1):10-38. doi: 10.2337/cd22-as01 [Crossref] [ Google Scholar]
- Muller IS, de Grauw WJ, van Gerwen WH, Bartelink ML, van Den Hoogen HJ, Rutten GE. Foot ulceration and lower limb amputation in type 2 diabetic patients in Dutch primary health care. Diabetes Care 2002; 25(3):570-4. doi: 10.2337/diacare.25.3.570 [Crossref] [ Google Scholar]
- Andreoli TE, Cecil RL, Carpenter CC, et al. Cecil Essentials of Medicine. Saunders; 2001.
- Meijer JW, Bosma E, Lefrandt JD, Links TP, Smit AJ, Stewart RE. Clinical diagnosis of diabetic polyneuropathy with the diabetic neuropathy symptom and diabetic neuropathy examination scores. Diabetes Care 2003; 26(3):697-701. doi: 10.2337/diacare.26.3.697 [Crossref] [ Google Scholar]
- Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res 2014; 80:21-35. doi: 10.1016/j.phrs.2013.12.005 [Crossref] [ Google Scholar]
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL. Diabetic neuropathy. Nat Rev Dis Primers 2019; 5(1):41. doi: 10.1038/s41572-019-0092-1 [Crossref] [ Google Scholar]
- Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes 1997; 46 Suppl 2:S54-7. doi: 10.2337/diab.46.2.s54 [Crossref] [ Google Scholar]
- Ropper AH, Samuels MA, Klein JP, Prasad S. Diseases of the peripheral nerves. In: Adams and Victor’s Principles of Neurology. 11th ed. New York, NY: McGraw-Hill Education; 2019.
- Madani SP, Larijani B, Erfani MH, Heshmat R. Comparison of clinical criteria with neurophysiologic findings of sural nerve in diagnosis of diabetic peripheral neuropathy. Iran J Diabetes Metab 2004;3(2):135-40. [Persian].
- Dumitru D, Amato AA, Zwarts MJ. Electrodiagnostic Medicine. Hanley & Belfus; 2002.
- Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2016; 79(3):629-61. doi: 10.1021/acs.jnatprod.5b01055 [Crossref] [ Google Scholar]
- Sobhani Z, Nami SR, Emami SA, Sahebkar A, Javadi B. Medicinal plants targeting cardiovascular diseases in view of Avicenna. Curr Pharm Des 2017; 23(17):2428-43. doi: 10.2174/1381612823666170215104101 [Crossref] [ Google Scholar]
- Shah G, Shri R, Panchal V, Sharma N, Singh B, Mann AS. Scientific basis for the therapeutic use of Cymbopogon citratus, Stapf (lemon grass). J Adv Pharm Technol Res 2011; 2(1):3-8. doi: 10.4103/2231-4040.79796 [Crossref] [ Google Scholar]
- Blumenthal M, Goldberg A, Brinckmann J. Herbal Medicine: Expanded Commission E Monographs. Integrative Medicine Communications, 2000.
- Ghassemi Dehkordi N, Sajjadi SE, Ghannadi A, Amanzadeh Y, Azadbakht M, Asghari GR, et al. Iranian herbal pharmacopoeia (IHP). Hakim Res J 2003;6(3):63-9. [Persian].
- López V, Martín S, Gómez-Serranillos MP, Carretero ME, Jäger AK, Calvo MI. Neuroprotective and neurological properties of Melissa officinalis. Neurochem Res 2009; 34(11):1955-61. doi: 10.1007/s11064-009-9981-0 [Crossref] [ Google Scholar]
- Sepand MR, Soodi M, Hajimehdipoor H, Soleimani M, Sahraei E. Comparison of neuroprotective effects of Melissa officinalis total extract and its acidic and non-acidic fractions against a β-induced toxicity. Iran J Pharm Res 2013; 12(2):415-23. [ Google Scholar]
- Awad R, Muhammad A, Durst T, Trudeau VL, Arnason JT. Bioassay-guided fractionation of lemon balm (Melissa officinalis L) using an in vitro measure of GABA transaminase activity. Phytother Res 2009; 23(8):1075-81. doi: 10.1002/ptr.2712 [Crossref] [ Google Scholar]
- Allahverdiyev A, Duran N, Ozguven M, Koltas S. Antiviral activity of the volatile oils of Melissa officinalis L against Herpes simplex virus type-2. Phytomedicine 2004; 11(7-8):657-61. doi: 10.1016/j.phymed.2003.07.014 [Crossref] [ Google Scholar]
- Ramanauskiene K, Raudonis R, Majiene D. Rosmarinic acid and Melissa officinalis extracts differently affect glioblastoma cells. Oxid Med Cell Longev 2016; 2016:1564257. doi: 10.1155/2016/1564257 [Crossref] [ Google Scholar]
- Stini E, Tsimogiannis D, Oreopoulou V. The valorisation of Melissa officinalis distillation by-products for the production of polyphenol-rich formulations. Molecules 2024; 29(2):377. doi: 10.3390/molecules29020377 [Crossref] [ Google Scholar]
- Petrisor G, Motelica L, Craciun LN, Oprea OC, Ficai D, Ficai A. Melissa officinalis: composition, pharmacological effects and derived release systems-a review. Int J Mol Sci 2022; 23(7):3591. doi: 10.3390/ijms23073591 [Crossref] [ Google Scholar]
- Shakeri A, Sahebkar A, Javadi B. Melissa officinalis L - A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 2016; 188:204-28. doi: 10.1016/j.jep.2016.05.010 [Crossref] [ Google Scholar]
- Park DH, Park SJ, Kim JM, Jung WY, Ryu JH. Subchronic administration of rosmarinic acid, a natural prolyl oligopeptidase inhibitor, enhances cognitive performances. Fitoterapia 2010; 81(6):644-8. doi: 10.1016/j.fitote.2010.03.010 [Crossref] [ Google Scholar]
- Falé PL, Borges C, Madeira PJ, Ascensão L, Araújo ME, Florêncio MH. Rosmarinic acid, scutellarein 4′-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“falso boldo”). Food Chem 2009; 114(3):798-805. doi: 10.1016/j.foodchem.2008.10.015 [Crossref] [ Google Scholar]
- Shimojo Y, Kosaka K, Noda Y, Shimizu T, Shirasawa T. Effect of rosmarinic acid in motor dysfunction and life span in a mouse model of familial amyotrophic lateral sclerosis. J Neurosci Res 2010; 88(4):896-904. doi: 10.1002/jnr.22242 [Crossref] [ Google Scholar]
- Asadi A, Shidfar F, Safari M, Hosseini AF, Fallah Huseini H, Heidari I. Efficacy of Melissa officinalis L (lemon balm) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a randomized, double-blind, clinical trial. Phytother Res 2019; 33(3):651-9. doi: 10.1002/ptr.6254 [Crossref] [ Google Scholar]
- Yang SJ, Min KW, Gupta SK, Park JY, Shivane VK, Pitale SU. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes Metab 2013; 15(5):410-6. doi: 10.1111/dom.12042 [Crossref] [ Google Scholar]
- Javadi B, Emami SA. Avicenna’s contribution to mechanisms of cardiovascular drugs. Iran J Basic Med Sci 2015; 18(8):721-2. [ Google Scholar]
- Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002; 76(3):560-8. doi: 10.1093/ajcn/76.3.560 [Crossref] [ Google Scholar]
- Naseri M, Rezaizadeh H, Choopani R, Anushirvani M. Review of Principles of Iranian Traditional Medicine. Tehran, Iran: Nashre Shahr; 2009. [Persian].
- Sarian MN, Ahmed QU, Mat So’ad SZ, Alhassan AM, Murugesu S, Perumal V. Antioxidant and antidiabetic effects of flavonoids: a structure-activity relationship-based study. Biomed Res Int 2017; 2017:8386065. doi: 10.1155/2017/8386065 [Crossref] [ Google Scholar]
- Miroliaei M, Khazaei S, Moshkelgosha S, Shirvani M. Inhibitory effects of lemon balm (Melissa officinalis, L) extract on the formation of advanced glycation end products. Food Chem 2011; 129(2):267-71. doi: 10.1016/j.foodchem.2011.04.039 [Crossref] [ Google Scholar]
- Naderifar M, Foroutan L, Kooh Khail A. Traditional medical practices used to treat diabetes. J Diabetes Nurs 2013;1(1):52-60. [Persian].
- Neri S, Calvagno S, Mauceri B, Misseri M, Tsami A, Vecchio C. Effects of antioxidants on postprandial oxidative stress and endothelial dysfunction in subjects with impaired glucose tolerance and type 2 diabetes. Eur J Nutr 2010; 49(7):409-16. doi: 10.1007/s00394-010-0099-6 [Crossref] [ Google Scholar]
- Bolkent S, Yanardag R, Karabulut-Bulan O, Yesilyaprak B. Protective role of Melissa officinalis L extract on liver of hyperlipidemic rats: a morphological and biochemical study. J Ethnopharmacol 2005; 99(3):391-8. doi: 10.1016/j.jep.2005.02.038 [Crossref] [ Google Scholar]
- Nayebi N, Esteghamati A, Meysamie A, Khalili N, Kamalinejad M, Emtiazy M. The effects of a Melissa officinalis L based product on metabolic parameters in patients with type 2 diabetes mellitus: a randomized double-blinded controlled clinical trial. J Complement Integr Med 2019; 16(3):20180088. doi: 10.1515/jcim-2018-0088 [Crossref] [ Google Scholar]
- Basar SN, Zaman R. An overview of badranjboya (Melissa officinalis). Int Res J Biol Sci 2013; 2(12):107-9. [ Google Scholar]
- Adinee J, Piri K, Karami O. Essential oil component in flower of lemon balm (Melissa officinalis L). Am J Biochem Biotechnol 2008; 4(3):277-8. [ Google Scholar]
- Aharizad S, Rahimi MH, Moghadam M, Mohebalipour N. Study of genetic diversity in lemon balm (Melissa officinalis L) populations based on morphological traits and essential oils content. Ann Biol Res 2012; 3(12):5748-53. [ Google Scholar]
- Ogbera AO, Dada O, Adeleye F, Jewo PI. Complementary and alternative medicine use in diabetes mellitus. West Afr J Med 2010; 29(3):158-62. doi: 10.4314/wajm.v29i3.68213 [Crossref] [ Google Scholar]
- Hasanein P, Riahi H. Antinociceptive and antihyperglycemic effects of Melissa officinalis essential oil in an experimental model of diabetes. Med Princ Pract 2015; 24(1):47-52. doi: 10.1159/000368755 [Crossref] [ Google Scholar]