Biomed Res Bull. 2(3):118-123.
doi: 10.34172/biomedrb.2024.18
Original Article
Sericin Has the Same Function With Natalizumab by Decreasing RORɤT and CD4 in a Multiple Sclerosis Animal Model
Zahra Sheikhalizadeh 1, *  , Ghazal Majidi 1, Zahra Hakimzadeh 1, Misagh Majidi 1
, Ghazal Majidi 1, Zahra Hakimzadeh 1, Misagh Majidi 1
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences,Tabriz, Iran
Abstract
Background:
Cytokines and T lymphocytes are key factors contributing to autoimmune disorders such as multiple sclerosis (MS). The main impact on T lymphocytes involves CD4+and CD8+cells, which degenerate the neurons by activating neuro-cytokines. The present study aimed to evaluate the effects of drug treatments in an MS animal model.
Methods:
Forty Balb/C mice were divided into four groups to induce the experimental autoimmune encephalomyelitis (EAE) model of MS. The central nervous system (CNS) cells were isolated from mice. The expression levels of RORɤT, IL-17, CD4, and CD8 were evaluated using real-time polymerase chain reaction (PCR). Simultaneously, the protein levels of RORɤT and IL-17 were evaluated by western blot.
Results:
The real-time PCR was performed to measure the expression of IL-17, RORɤT, CD8, and CD4. The results indicated that IL-17 expression decreased in the three drug-treated groups compared to the control (non-drug) group. Among the three-drug groups, natalizumab significantly decreased IL-17 expression more than the other treatments. Additionally, RORɤT expression decreased in all drug-treated groups, with sericin exhibiting the same effect as natalizumab and INF-B in decreasing RORɤT expression. The lowest expression of CD8 was observed in the natalizumab group. Importantly, CD4 expression decreased significantly in all three drug-treated groups, with sericin and natalizumab showing similar effects.
Conclusion:
The present study concluded that sericin is not a Food and Drug Administration (FDA)-approved drug for MS, but it has the same function in reducing the main inflammatory cytokines and CD4 levels in MS. This suggests that sericin can become an FDA-approved drug for treating MS in the future.
Keywords: Sericin, Natalizumab, RORɤT, Multiple sclerosis, Animal model
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was self-funded by the authors and received no external financial support from any funding organization.
Introduction
Multiple sclerosis (MS) is a neurodegenerative, inflammatory, immune-mediated disorder of the central nervous system (CNS) that leads to neural cell loss and/or neuroaxonal dystrophy, which predominantly affects young adulthood.1-4 MS is classified into four different forms: Relapsing/remitting MS (RRMS) which affects 85% of all MS patients and involves relapses followed by periods of remission, primary progressive MS (PPMS), affecting 8%–10% of patients and characterized by progressive neurologic decline, secondary progressive MS (SPMS), which develops gradually following a diagnosis of RRMS, and progressive relapsing MS (PRMS), which affects less than 5% of patients and is marked by overlapping relapses.3-8 While the pathophysiology and etiology of MS remain unknown, genetic, infectious (e.g., Epstein–Barr virus), nutritional (e.g., vitamin D deficiency), and environmental factors are thought to play a crucial role in initiating and progressing the disease by inducing an adaptive, lymphocyte-mediated autoimmune response against the CNS.5-9 Infiltrating immune cells secrete matrix metalloproteinase, nitric oxide, and pro-inflammatory cytokines, leading to the destruction of myelin sheath.10-12
Currently, there are nine classes of disease-modifying therapy treatments (DMTs) available, 20 approved DMTs in the United States, each with different mechanisms of action, including immunomodulatory agents (e.g., interferons and glatiramer acetate), sphingosine-1-phosphate receptor modulators (e.g., fingolimod, siponimod, ozanimod, and ponesimod), fumarates (e.g., dimethyl fumarate, diroximel fumarate, and monomethyl fumarate), cladribine, teriflunomide, natalizumab, alemtuzumab, and lymphocyte-depleting agents (e.g., ocrelizumab and ofatumumab).13-15 Additionally, there is ongoing research into immune-modulating strategies such as DNA vaccines, altered peptide ligands, and stem cells for managing MS.
Sericin is a macromolecular protein that is usually discarded in the textile industry, but due to its biocompatibility, biodegradability, and immunocompatibility, it can be used as a drug carrier and even independently as a treatment for various conditions. Studies have demonstrated that adding sericin does not induce cytotoxicity in several mammalian cell line cultures and thus can be used as a supplement for cell cultures.6 Furthermore, sericin can influence neuroregeneration and has unique properties that allow it to function as a drug delivery vehicle, enabling sustained drug release and thus reducing the need for repeated treatments, as well as minimizing the risk of drug toxicity.7
In a survey investigating silk fibroin stability in both in vitro and in vivo environments on two neuroinflammatory models mimicking stroke and Alzheimer’s disease, it was found to be minimally degraded, thus showing great potential as a drug delivery vehicle. However, a concern arises regarding the risk of the product remaining at the site for a prolonged period.8 Sericin, which is eliminated during the silk degumming process, has been identified as a potential biomaterial due to its anti-inflammatory, antioxidant, immune compatibility, and antibacterial properties.13-15 Therefore, this protein has been recognized for numerous pharmaceutical and biomedical applications, including neuro-protection, anti-diabetic, anti-hypertensive, anti-tumor, anti-wrinkle, anti-aging, and wound-healing effects.16-22 The efficacy of silk sericin in suppressing pro-inflammatory cytokine production has been reported, but its direct role in any anti-inflammatory processes remains understudied.23,24
Cytokines are secreted proteins with specific effects on cellular interaction and communication and can be predominantly produced by T helper cells and macrophages. Despite the unidentified underlying cause of MS, several studies have highlighted cytokines as key agents in MS pathogenesis.25 Pro-inflammatory cytokines are markedly increased before the onset of the disease and during active phases, and they decrease during the remission period. It is speculated that part of the MS inflammatory process is stimulated by cytokine infiltration of the Blood Brain Barrier, resulting in the secretion of cytokines and chemokines, especially Th1, by astrocytes, macrophages, microglia, and T cells in active lesions within cerebral tissue.22 This study aimed to evaluate the expression of RORɤT, IL-17, and T lymphocyte markers in an MS animal model.
Materials and Methods
Animals and Experimental Autoimmune Encephalomyelitis Model Induction
Forty male C57BL/6 mice (8–10 weeks old) were purchased from Mega Teb Bakhtar (Tabriz, Iran). The animals were nourished and treated following the ethical guidelines adopted by the Ethics Committee of the Tabriz University of Medical Sciences. The mice were divided into four groups: Control group (n = 10), sericin group (n = 10), INF-B group (n = 10), and natalizumab group (n = 10). To induce the experimental autoimmune encephalomyelitis (EAE) model, the MOG35–55 peptide (Faraz Teb Co., Tabriz, Iran) was synthesized by Proteimax. The mice were immunized subcutaneously with 150 μg of MOG35–55 peptides emulsified in complete Freund’s adjuvant (CFA) containing 5 mg/mL of mycobacteria. Additionally, the mice received two doses of 200 ng of Bordetella pertussis toxin (Sigma) intraperitoneally, at 0 and 48 hours after immunization. Clinical assessments were performed according to the following criteria:
0. No disease
1. limp tail
2. Weak/partially paralyzed hind legs
3. Completely paralyzed hind legs
4. Complete hind and partial front leg paralysis
5. Complete paralysis or death.
Isolation of Central Nervous System-Infiltrating Cells
Mice were anesthetized using ketamine and xylazine and then perfused with 10 mL of saline solution. The brain and cervical spinal cords were excised in 4 mL of RPMI (Sigma) and incubated. The suspensions were washed in RPMI, then centrifuged at 450 × g for 15 minutes at 4 °C. Forty-five minutes later, the cells were resuspended and centrifuged at 950 × g for 20 minutes. After centrifugation, the ring containing mononuclear cells was collected, washed with RPMI, and centrifuged at 1500 rpm for 10 minutes. This process was repeated twice.
Real-Time PCR and RNA Extraction
The cells were collected, and total RNA was extracted. The expression of IL-17, RORɤT, CD8, and CD4 was evaluated using real-time polymerase chain reaction (PCR). The PCR mix contained 1 μ of RNA, 1 μL of 5 mM dNTP, 2.5 μL of Taq polymerase, 1.2 μL of 50 mM MgCl2, and 1 μL of each primer (Table 1). The PCR condition included: initial denaturation at 94 °C for 5 minutes, followed by 35 cycles of denaturation at 94 °C for 30 seconds, annealing at 56 °C for 30 seconds, extension at 72 °C for 40 minutes, and a final elongation cycle at 72 °C for 10 minutes (Corbett 6000 real-time PCR). Beta-actin was used as a control.
Table 1.
Primer list for using Real Time PCR
|
Gene
|
Sequence
|
| RORɤT |
Forward 3- AATTCGGGGTACTTCACC-5
Revers 3- AATTTCGGATCGGTACCC-5 |
| IL-17 |
Forward 3- ATCATACCTAGGTTCGGC-5
Revers 3- GGAAATCGGTTCTCCCTA-5 |
| CD4 |
Forward 3- ATCGAATTTCGGCGTACC-5
Revers 3- CGATCAATTATTCGGCCG-5 |
| CD8 |
Forward 3- ATCGGTACCCATTTCGGA-5
Revers 3- ATACCGATCATGGCCGTT-5 |
| β-actin |
Forward 3- CGGTTTCGGATCCCTAAA-5
Revers 3- GATAAGGTACCCTTTCGC-5 |
Western Blot Analyzing
Cells were lysed in a buffer composed of 50 mm Tris, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM NaF, 1 mM Na3VO4, 2 mM Na4P2O7, 1 mM phenylmethanesulfonyl fluoride (PMSF) and ‘complete’ protease inhibitor cocktail tablets (Roche, Basel, Switzerland). Cell debris was removed by centrifugation, and the protein concentration was quantified using a protein assay (Thermo Scientific NanoDrop 1000 UV–vis Spectrophotometer). Proteins were boiled in SDS gel-loading buffer with 10% β-mercaptoethanol. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyscreen polyvinylidene fluoride (PVDF) membranes (PerkinElmer, Boston, MA, USA). Membranes were blocked with 5% (w/v) non-fat dry milk and 1% (v/v) Tween 20 in PBS for 1 hour at room temperature and then incubated overnight with commercially available anti-IL-17 and anti-RORɤT antibodies (1:1000, Abcam) at 4 °C. Detection was performed using electrochemiluminescence (ECL), and blots were quantified by densitometry using the image analysis program (Amercontrol Biosciences, San Francisco, CA, USA).
Statistical Analysis
Data were expressed as mean ± standard error. Comparisons between groups were made by student’s t-test or one-way ANOVA, and the significance level was set at P < 0.001.
Results
Sericin Decreases CD4 and ROR ɤ T Expression
Real-time PCR was performed to determine the expression levels of IL-17, RORɤT, CD8, and CD4. The results indicated that IL-17 expression was reduced in all three drug groups compared to the control (non-drug) group. Among the three drug groups, natalizumab exhibited the most significant reduction in IL-17 expression. RORɤT expression was decreased in all drug groups, but sericin had a similar effect to natalizumab and INF-B in decreasing its expression. The lowest CD8 expression was observed in the natalizumab group compared to the other groups. The most significant finding was that CD4 expression decreased significantly in all three drug groups, with sericin and natalizumab playing similar roles in this reduction (Figure 1).
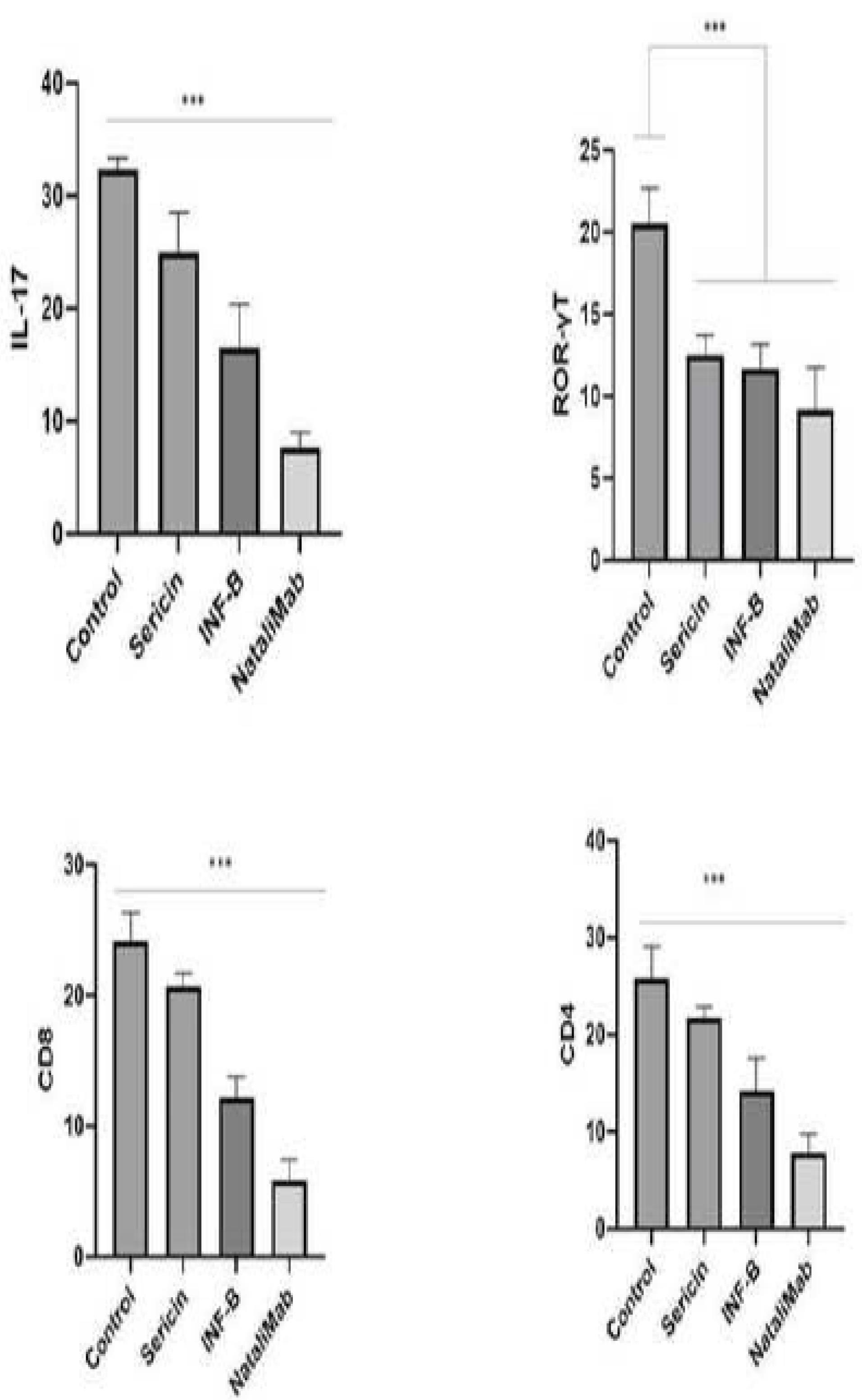
Figure 1.
The Real-Time PCR Results for IL-17, RORɤT, CD, and CD8 in Four Groups of the EAE Model. Note. EAE: Experimental autoimmune encephalomyelitis; PCR: Polymerase chain reaction. *** P<0.0001
.
The Real-Time PCR Results for IL-17, RORɤT, CD, and CD8 in Four Groups of the EAE Model. Note. EAE: Experimental autoimmune encephalomyelitis; PCR: Polymerase chain reaction. *** P<0.0001
Decreasing the Expression of Protein Levels of ROR ɤ T via Sericin and Natalizumab
Western blot analysis was conducted to evaluate the protein levels of IL-17 and RORɤT in cells isolated from mice of the four groups. The results showed that RORɤT levels decreased in the sericin and natalizumab groups compared to the control group. IL-17 did not exhibit a significant decrease, except in the natalizumab group (Figure 2).
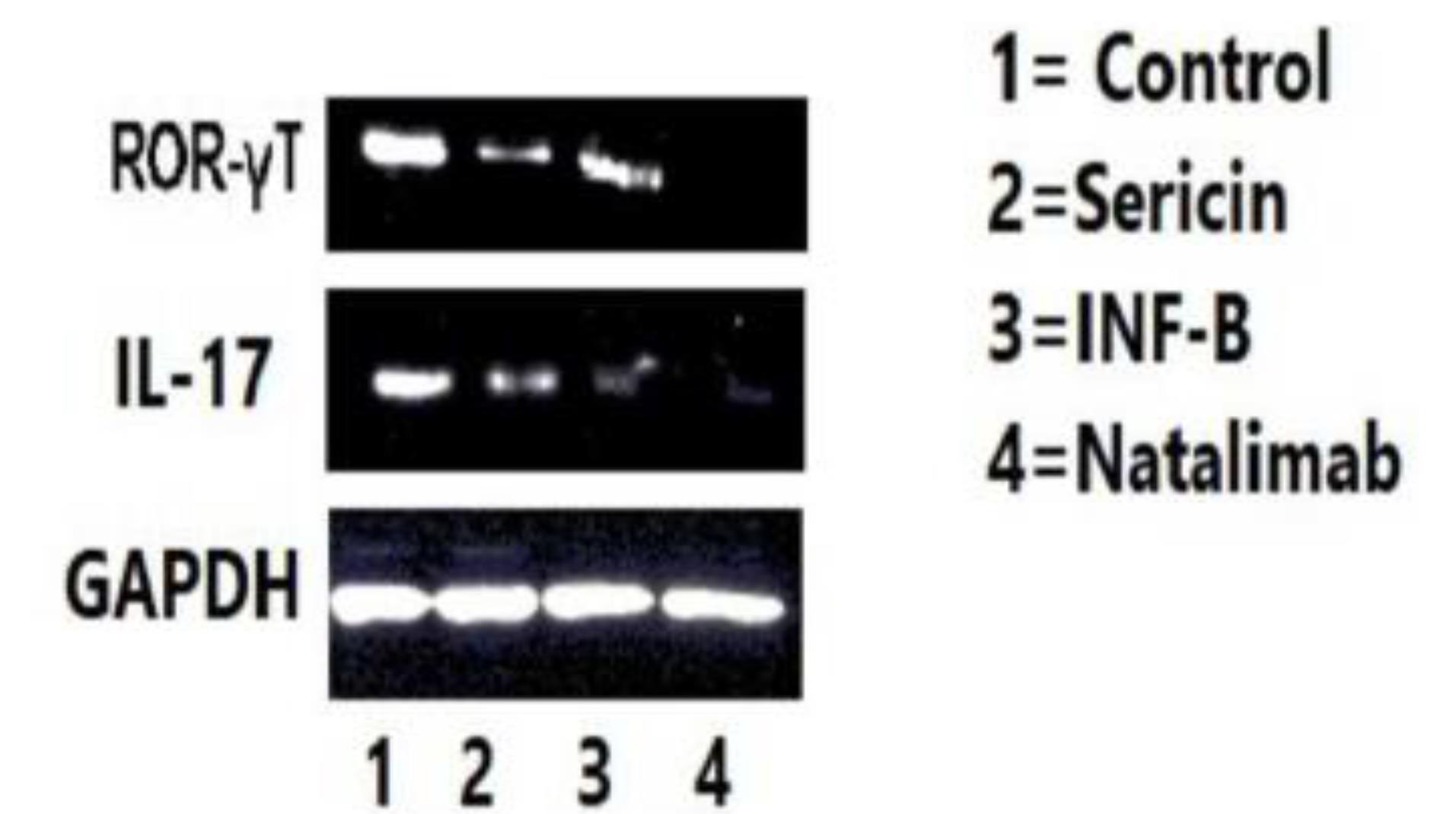
Figure 2.
The Protein Level Expression in Four Groups
.
The Protein Level Expression in Four Groups
Discussion
The present study focused on the effects of three drugs on RORɤT, CD4, CD8, and IL-17 expression in the EAE model of MS. Recent studies have demonstrated that sericin can inhibit neuroinflammatory responses in the prefrontal cortex and hippocampus by decreasing concentrations of TNF-α, NF-κB, and IL-1β while suppressing the mitochondrial-dependent apoptosis pathway.26 Additionally, the neuroprotective benefits of sericin have been observed in peripheral neuropathy in diabetic patients and Alzheimer’s disease model.27 Sericin has also been shown to protect against memory impairment caused by acute sleep deprivation by enhancing hippocampal synaptic protein concentrations and reducing neuroinflammation and oxidative stress levels in mice.28 Our results suggest that sericin can also decrease inflammatory cytokines such as IL-17, although this reduction is not as pronounced as in other drug treatments.
Th1 cytokines such as IL-1B, IL-6, TNF-a, and their specific cytokine IFN-γ can induce MS animal models such as EAE. Initially, it was assumed that Th1 cytokines have a similar effect on MS due to the presence of IFN-γ and IL-12 levels in MS lesions and cerebrospinal fluid (CSF). However, further evidence has questioned their importance, as IFN-γ -/- and IL-12 -/- mice were still susceptible to EAE, while IL-23 -/- models showed resistance to EAE. Since cytokine is a key promoter of Th17 cells, this finding opened new areas of research for researchers to explore.29,30 The present study interestingly showed that sericin has the same function as natalizumab and INF-B in decreasing RORɤT expression. IL-1β, combined with IL-23, can expand pathogenic Th17 cells, which regulate GM-CSF derived from TH17. IL- 1β and GM-CSF can create a feedback cycle that exacerbates CNS inflammation and results in neural and myelin injury.21 However, other studies have demonstrated the regulatory effects of these cytokines on oligodendrocyte differentiation and neural remyelination, suggesting a dual effect similar to TNF. This dual effect indicates that treatment with IL-1 blockers should be approached with caution.31
Numerous studies have explored cytokine effects and the different activated pathways involved in MS pathogenesis, but remains to be discovered to hopefully help with early diagnosis, symptom relief, and eventually reversing the debilitating side effects of MS. Several studies have also examined the anti-inflammatory mechanisms of sericin. For example, Sun et al published a study highlighting sericin’s inhibitory effect on lipopolysaccharide (LPS)-induced inflammation, revealing its genome-wide effect by inhibiting inflammatory cytokine genes such as IL-1B, IL-6, IL-18, and IFN. Moreover, sericin exerted an anti-inflammatory effect by downregulating the NF-κB pathway activated by LPS.32 Aramwit et al investigated the anti-inflammatory effects of sericin in several subsequent studies. In one of the studies, rat wounds were treated with silk sericin, and the levels of pro-inflammatory mediators such as TNF-α and IL-1B were found to be significantly lower compared to the control group.33 In 2015, another study was conducted to evaluate sericin’s anti-inflammatory effect by inducing inflammation and edema in rats. The results revealed that sericin successfully decreased inflammation in these models.34 Additionally, a study conducted in 2023 assessed the effects of sericin-coated polymeric fibers on psoriasis rat models. This treatment, via the mTOR signaling pathway, could reduce TNF-α and Wnt levels, inhibit epidermal proliferation, and decrease TGF-Β and IL-1B, ultimately reducing IL-17 production. However, no significant changes were found in IL-21 and IL-22 levels.5 Furthermore, Deenonpoe et al showed that exposure to naringin and sericin significantly lowered the production of cytokines such as TNF-α, IL-6, IL-12p40, and IL-23 in human peripheral blood mononuclear cells.35,36
Sericin can be used independently in several neurological conditions. In a study analyzing the effects of sericin on memory and sociability impairments evoked by transient global cerebral ischemia, it was found that while ischemia increased IL-6 and TNF-α levels and decreased IL-10 (an anti-inflammatory cytokine), sericin treatment improved memory retention and social interactions. This was attributed to sericin’s ability to reduce oxidative stress and apoptotic signals in the hippocampus. Furthermore, sericin can regulate the inflammatory response by decreasing IL-6 and TNF-α levels while increasing IL-10 levels, thus exerting a neuroprotective impact against ischemic injury.37 Similarly, a 2021 study demonstrated that sericin had a positive effect on spatial and social memory in aged mice by regulating inflammatory cytokines.38 Wang et al explained sericin’s anti-apoptotic mechanism in the hippocampus of rat models of diabetes mellitus, where sericin treatment led to increased p-Akt and NF-κB protein and mRNA expression levels.39,40
Conclusion
The present study concluded that although sericin is not yet a Food and Drug Administration (FDA)-approved drug for MS, it exhibits similar functions to existing drugs in decreasing main inflammatory cytokines and CD4 levels in MS, which can cause sericin to be developed into an FDA-approved drug for treating MS in the future.
Authors’ Contribution
Conceptualization: Zahra Sheikhalizadeh.
Data curation: Ghazal Majidi, Zahra Hakimzadeh.
Formal analysis: Misagh Majidi.
Investigation: Zahra Sheikhalizadeh.
Methodology: Zahra Hakimzadeh, Misagh Majidi.
Project administration: Zahra Sheikhalizadeh, Ghazal Majidi.
Resources: Zahra Sheikhalizadeh.
Software: Ghazal Majidi, Zahra Hakimzadeh.
Supervision: Zahra Sheikhalizadeh.
Validation: Ghazal Majidi.
Visualization: Zahra Sheikhalizadeh.
Writing–original draft: Ghazal Majidi.
Writing–review & editing: Zahra Sheikhalizadeh.
Competing Interests
None.
Ethical Approval
This study was approved by the Ethics Committee of the Tabriz University of Medical Sciences (Ethics approval no. IR.TBZMED.REC.1398.977)
Acknowledgements
This study was conducted at the Immunology Research Center of Tabriz Medical University and the Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences.
References
- Rafiee Zadeh A, Ghadimi K, Ataei A, Askari M, Sheikhinia N, Tavoosi N. Mechanism and adverse effects of multiple sclerosis drugs: a review article Part 2. Int J Physiol Pathophysiol Pharmacol 2019; 11(4):105-14. [ Google Scholar]
- Wiendl H, Gold R, Berger T, Derfuss T, Linker R, Mäurer M. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord 2021; 14:17562864211039648. doi: 10.1177/17562864211039648 [Crossref] [ Google Scholar]
- Belalzadeh M, Anguti F, Vedadi S, Deljavan Ghodrati M. Investigation of biochemical factors in multiple sclerosis patients with fingolimod and natalizumab drugs. Biomed Res Bull 2023; 1(1):7-10. doi: 10.34172/biomedrb.2023.03 [Crossref] [ Google Scholar]
- Cramer E. Ueber die bestandtheile der seide. J Prakt Chem 1865; 96:76-98. [ Google Scholar]
- Aramwit P, Siritientong T, Srichana T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag Res 2012; 30(3):217-24. doi: 10.1177/0734242x11404733 [Crossref] [ Google Scholar]
- Liu J, Shi L, Deng Y, Zou M, Cai B, Song Y. Silk sericin-based materials for biomedical applications. Biomaterials 2022; 287:121638. doi: 10.1016/j.biomaterials.2022.121638 [Crossref] [ Google Scholar]
- Zhang YQ. Applications of natural silk protein sericin in biomaterials. Biotechnol Adv 2002; 20(2):91-100. doi: 10.1016/s0734-9750(02)00003-4 [Crossref] [ Google Scholar]
- Kunz RI, Brancalhão RM, Ribeiro LF, Natali MR. Silkworm sericin: properties and biomedical applications. Biomed Res Int 2016; 2016:8175701. doi: 10.1155/2016/8175701 [Crossref] [ Google Scholar]
- Suryawanshi R, Kanoujia J, Parashar P, Saraf SA. Sericin: a versatile protein biopolymer with therapeutic significance. Curr Pharm Des 2020; 26(42):5414-29. doi: 10.2174/1381612826666200612165253 [Crossref] [ Google Scholar]
- Silva AS, Costa EC, Reis S, Spencer C, Calhelha RC, Miguel SP. Silk sericin: a promising sustainable biomaterial for biomedical and pharmaceutical applications. Polymers (Basel) 2022; 14(22):4931. doi: 10.3390/polym14224931 [Crossref] [ Google Scholar]
- Kundu SC, Dash BC, Dash R, Kaplan DL. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog Polym Sci 2008; 33(10):998-1012. doi: 10.1016/j.progpolymsci.2008.08.002 [Crossref] [ Google Scholar]
- Rocha LK, Favaro LIL, Rios AC, Silva EC, Silva WF, Stigliani TP. Sericin from Bombyx mori cocoons Part I: extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem 2017; 61:163-77. doi: 10.1016/j.procbio.2017.06.019 [Crossref] [ Google Scholar]
- Ghosh S, Rao RS, Shwetha Nambiar K, Haragannavar VC, Augustine D, Sowmya SV. Sericin, a dietary additive: mini review. J Med Radiol Pathol Surg 2017; 4(2):13-17. [ Google Scholar]
- Elahi M, Ali S, Tahir HM, Mushtaq R, Bhatti MF. Sericin and fibroin nanoparticles—natural product for cancer therapy: a comprehensive review. Int J Polym Mater Polym Biomater 2021; 70(4):256-69. doi: 10.1080/00914037.2019.1706515 [Crossref] [ Google Scholar]
- Rahimpour S, Jabbari H, Yousofi H, Fathi A, Mahmoodi S, Jafarian MJ. Regulatory effect of sericin protein in inflammatory pathways; a comprehensive review. Pathol Res Pract 2023; 243:154369. doi: 10.1016/j.prp.2023.154369 [Crossref] [ Google Scholar]
- Baptista-Silva S, Borges S, Costa-Pinto AR, Costa R, Amorim M, Dias JR. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater Sci Eng 2021; 7(4):1573-86. doi: 10.1021/acsbiomaterials.0c01745 [Crossref] [ Google Scholar]
- Yang C, Li S, Huang X, Chen X, Shan H, Chen X. Silk fibroin hydrogels could be therapeutic biomaterials for neurological diseases. Oxid Med Cell Longev 2022; 2022:2076680. doi: 10.1155/2022/2076680 [Crossref] [ Google Scholar]
- Yonesi M, Ramos M, Ramirez-Castillejo C, Fernández-Serra R, Panetsos F, Belarra A. Resistance to degradation of silk fibroin hydrogels exposed to neuroinflammatory environments. Polymers (Basel) 2023; 15(11):2491. doi: 10.3390/polym15112491 [Crossref] [ Google Scholar]
- Xu S, Yang Q, Wang R, Tian C, Ji Y, Tan H. Genetically engineered pH-responsive silk sericin nanospheres with efficient therapeutic effect on ulcerative colitis. Acta Biomater 2022; 144:81-95. doi: 10.1016/j.actbio.2022.03.012 [Crossref] [ Google Scholar]
- Yalcin E, Kara G, Celik E, Pinarli FA, Saylam G, Sucularli C. Preparation and characterization of novel albumin-sericin nanoparticles as siRNA delivery vehicle for laryngeal cancer treatment. Prep Biochem Biotechnol 2019; 49(7):659-70. doi: 10.1080/10826068.2019.1599395 [Crossref] [ Google Scholar]
- Jiang JP, Liu XY, Zhao F, Zhu X, Li XY, Niu XG. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen Res 2020; 15(5):959-68. doi: 10.4103/1673-5374.268974 [Crossref] [ Google Scholar]
- Khosropanah MH, Majidi Zolbin M, Kajbafzadeh AM, Amani L, Harririan I, Azimzadeh A. Evaluation and comparison of the effects of mature silkworm (Bombyx mori) and silkworm pupae extracts on Schwann cell proliferation and axon growth: an in vitro study. Iran J Pharm Res 2022; 21(1):e133552. doi: 10.5812/ijpr-133552 [Crossref] [ Google Scholar]
- Banagozar Mohammadi A, Torbati M, Farajdokht F, Sadigh-Eteghad S, Fazljou SM, Vatandoust SM. Sericin alleviates restraint stress induced depressive- and anxiety-like behaviors via modulation of oxidative stress, neuroinflammation and apoptosis in the prefrontal cortex and hippocampus. Brain Res 2019; 1715:47-56. doi: 10.1016/j.brainres.2019.03.020 [Crossref] [ Google Scholar]
- Mahmoudi J, Hosseini L, Sadigh-Eteghad S, Farajdokht F, Vatandoust SM, Ziaee M. Sericin alleviates thermal stress induced anxiety-like behavior and cognitive impairment through regulation of oxidative stress, apoptosis, and heat-shock protein-70 in the hippocampus. Neurochem Res 2021; 46(9):2307-16. doi: 10.1007/s11064-021-03370-6 [Crossref] [ Google Scholar]
- Chen Z, He Y, Song C, Dong Z, Su Z, Xue J. Sericin can reduce hippocampal neuronal apoptosis by activating the Akt signal transduction pathway in a rat model of diabetes mellitus. Neural Regen Res 2012; 7(3):197-201. doi: 10.3969/j.issn.1673-5374.2012.03.007 [Crossref] [ Google Scholar]
- Vatandoust SM, Meftahi GH. The effect of sericin on the cognitive impairment, depression, and anxiety caused by learned helplessness in male mice. J Mol Neurosci 2022; 72(5):963-74. doi: 10.1007/s12031-022-01982-3 [Crossref] [ Google Scholar]
- Peera KU, Yellamma KU. Sericin as a cholinergic modulator in Alzheimer’s disease induced rat. Int J Pharm Pharm Sci 2015; 7(4):108-12. [ Google Scholar]
- Peera K, Thulasi M, Shantkriti S, Reddy MS. Silk protein-sericin induced oxidative lipid peroxidation alterations in Alzheimer’s disease rat model. Indian Journal of Comparative Animal Physiology 2019; 37:60-72. [ Google Scholar]
- Yellamma K. Silk protein, sericin as a cognitive enhancer in Alzheimer’s disease. J Alzheimers Dis Parkinsonism 2014; 4(5):163. doi: 10.4172/2161-0460.1000163 [Crossref] [ Google Scholar]
- Park D, Lee SH, Choi YJ, Bae DK, Yang YH, Yang G. Improving effect of silk peptides on the cognitive function of rats with aging brain facilitated by D-galactose. Korean Soc Appl Pharmacol 2011; 19(2):224-30. [ Google Scholar]
- Kim TK, Park D, Yeon S, Lee SH, Choi YJ, Bae DK. Tyrosine-fortified silk amino acids improve physical function of Parkinson’s disease rats. Food Sci Biotechnol 2011; 20(1):79-84. doi: 10.1007/s10068-011-0011-z [Crossref] [ Google Scholar]
- Sun Y, Shi W, Zhang Q, Guo H, Dong Z, Zhao P. Multi-omics integration to reveal the mechanism of sericin inhibiting LPS-induced inflammation. Int J Mol Sci 2022; 24(1):259. doi: 10.3390/ijms24010259 [Crossref] [ Google Scholar]
- Aramwit P, Kanokpanont S, De-Eknamkul W, Srichana T. Monitoring of inflammatory mediators induced by silk sericin. J Biosci Bioeng 2009; 107(5):556-61. doi: 10.1016/j.jbiosc.2008.12.012 [Crossref] [ Google Scholar]
- Aramwit P, Towiwat P, Srichana T. Anti-inflammatory potential of silk sericin. Nat Prod Commun 2013; 8(4):501-4. [ Google Scholar]
- Aramwit P, Fongsodsri K, Tuentam K, Reamtong O, Thiangtrongjit T, Kanjanapruthipong T. Sericin coated thin polymeric films reduce keratinocyte proliferation via the mTOR pathway and epidermal inflammation through IL17 signaling in psoriasis rat model. Sci Rep 2023; 13(1):12133. doi: 10.1038/s41598-023-39218-y [Crossref] [ Google Scholar]
- Deenonpoe R, Prayong P, Thippamom N, Meephansan J, Na-Bangchang K. Anti-inflammatory effect of naringin and sericin combination on human peripheral blood mononuclear cells (hPBMCs) from patient with psoriasis. BMC Complement Altern Med 2019; 19(1):168. doi: 10.1186/s12906-019-2535-3 [Crossref] [ Google Scholar]
- Vatandoust SM, Mahmoudi J, Oryan S, Farajdokht F, Sadigh-Eteghad S, Sandoghchian Shotorbani S. Sericin improves memory and sociability impairments evoked by transient global cerebral ischemia through suppression of hippocampal oxidative stress, inflammation, and apoptosis. Chin J Physiol 2023; 66(4):209-19. doi: 10.4103/cjop.CJOP-D-23-00006 [Crossref] [ Google Scholar]
- Seyedaghamiri F, Farajdokht F, Vatandoust SM, Mahmoudi J, Khabbaz A, Sadigh-Eteghad S. Sericin modulates learning and memory behaviors by tuning of antioxidant, inflammatory, and apoptotic markers in the hippocampus of aged mice. Mol Biol Rep 2021; 48(2):1371-82. doi: 10.1007/s11033-021-06195-2 [Crossref] [ Google Scholar]
- Wang Z, Wang J, Jin Y, Luo Z, Yang W, Xie H. A neuroprotective sericin hydrogel as an effective neuronal cell carrier for the repair of ischemic stroke. ACS Appl Mater Interfaces 2015; 7(44):24629-40. doi: 10.1021/acsami.5b06804 [Crossref] [ Google Scholar]
- Wang Z, Wang J, Jin Y, Luo Z, Yang W, Xie H. Correction to “a neuroprotective sericin hydrogel as an effective neuronal cell carrier for the repair of ischemic stroke”. ACS Appl Mater Interfaces 2023; 15(17):21719. doi: 10.1021/acsami.3c05003 [Crossref] [ Google Scholar]