Biomed Res Bull. 2(2):75-78.
doi: 10.34172/biomedrb.2024.12
Review Article
Enhancing Antibody Uptake: Strategies for Improved Immunotherapy Efficacy
Shiva Alipour 1, 2, 3  , Rozita Abolhasan 1, Leili Aghebati-Maleki 1, *
, Rozita Abolhasan 1, Leili Aghebati-Maleki 1, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Antibodies play a starring role in immunotherapy for numerous tumors and neurodegenerative disorders such as Alzheimer’s disease (AD). Nevertheless, low uptake of antibodies, especially monoclonal antibody (mAb) therapy, in solid tumors limits the effectiveness of these therapeutics due to tumor-specific physiological features. This review scrutinizes the significance of antibody uptake and discusses methods to improve it because enhancing their uptake is crucial for better therapeutic efficacy. Different factors, including antigen expression, antibody-antigen affinity, receptors, vascular permeability, and tumor size, influence antibody uptake. Hence, several strategies have been investigated to overcome these obstacles, including magnetic resonance-guided focused ultrasound (MR-FUS) treatment, bivalent brain shuttles, nanomaterials such as zeolite nanocrystals, and fibrosis pathway antagonists such as relaxin-2 by reducing extracellular matrix (ECM) accumulation and improving antibody transport in solid tumors. Furthermore, cholesterol-sequestering agents and hyaluronidase have been examined to enhance antibody uptake via controlling receptor trafficking and removing hyaluronan barriers. Combination therapies, including molecular and external radiotherapy or mAb immunotherapy and vasoactive immunoconjugates, have demonstrated augmented uptake. In conclusion, increasing antibody uptake is essential for boosting immunotherapy’s effectiveness in various disorders, and various strategies lead to encouraging outcomes to get beyond the drawbacks of limited antibody uptake. Gaining knowledge about them and putting these promising tactics into practice can help improve the results of cancer treatment.
Keywords: Antibody, Antibody uptake, Immunotherapy, Tumor
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was supported by Research Vice- Chancellor at Tabriz University of Medical Sciences, Iran. [Grant No. 74289].
Importance of Antibody Uptake
Antibodies are used for the immunotherapy of a variety of cancers1,2 and neurodegenerative diseases such as Alzheimer’s,3 and they are increasingly being tested as imaging applications in the diagnosis and monitoring of therapeutic effectiveness.4 Antibodies are able to specifically bind to a target antigen with high affinity and specificity. They work in numerous manners to produce therapeutic benefits. By attaching to cell membrane-associated receptors and either blocking their function or increasing their degradation, or by adhering to and neutralizing soluble growth factors, antibodies can counteract tumor growth pathways.5 Cell membrane receptor-binding antibodies can also attract immune effector cells by binding to fragment crystallizable (Fc) gamma receptors, which results in the death of tumor cells by either antibody-dependent phagocytosis by monocytes and macrophages or cellular cytotoxicity by natural killer cells.6
Nevertheless, the large size of these macromolecules and quick binding in the tumor tissue, along with the abnormal physiology of tumors, cause slow localization and heterogeneous uptake.7 The development of monoclonal antibody (mAb) therapy for solid tumors has received substantial funding; nonetheless, the level of clinical success has been limited, and this low effectiveness of mAb in solid tumors is probably related to particular features of tumor physiology that lead to the low uptake of antibodies. For instance, solid tumors delay the convective and diffusive transport of mAbs into tumors due to their dense extracellular matrix (ECM) and abnormal vasculature.8
Therefore, the major limitation for their better efficacy is the low uptake and the low absolute amount reaching the target. Various factors can affect the uptake of antibodies in the target environment, including the affinity of antibody to antigen,9 expression of antigens,10 receptors,11 vascular permeability,12 transcapillary pressure gradient,13 tumor size and age,14 and temperature of the environment.15 High expression of antigens and their quick turnover can cause perivascular cells to bind to and remove a large number of extravasated mAbs in the case of mAbs directed against cellular antigens, restricting the distribution of mAb to tumor areas.8
Strategies Affecting Antibody Uptake
In recent years, many studies have worked on strategies that affect antibody uptake. This section focuses on reviewing the results of these studies.
Brighi et al assessed the effect of magnetic resonance-guided focused ultrasound (MR-FUS) on antibody delivery to nonenhancing high-grade glioma (HGG). The therapeutic targeting of the HGG cell population is significantly hindered by the heterogeneous vasculature morphology of the tumor and the presence of an intact blood-brain barrier (BBB). In this study, structural MR imaging showed that the FUS treatment locally induced further opening and temporary disruption in the BBB and as a result, increased the amount of 89Zr-radiolabelled antibody uptake in the nonenhancing HGG tissue while leaving untargeted regions unaffected.16
In another study, the researchers designed a novel bivalent brain shuttle to overcome the BBB, immunotherapy, and antibody-based diagnostics by receptor-mediated transcytosis for Alzheimer’s disease (AD). Thus, they recombinantly attached two single-chain variable fragments of the transferrin receptor antibody to the C-terminal end of mAb158 (anti-Amyloidβ protofibrils) light chains, which are rolled in the pathogenesis of AD. They demonstrated that recombinant design improved receptor-mediated mAb158 brain uptake and Aβ immunotherapy. Thus, it can be used as a positron emission tomography radioligand for the point-of-care diagnosis and assessment of treatment effects in AD.17
Likewise, Chen et al evaluated the effect of cholesterol-sequestering on the improvement of tumor uptake and therapeutic efficacy of cetuximab (the anti-epidermal growth factor receptor [EGFR] antibody) in human carcinoma cell lines.18 They identified that nystatin cholesterol sequestration improved cetuximab uptake in carcinoma cells by regulating EGFR trafficking/turnover and simplifying a switch from lipid rafts to clathrin-mediated endocytosis. Therefore, cetuximab and nystatin combination therapy selectively enhanced cetuximab uptake by tumor tissues.18
Some studies have used nanomaterials to enhance antibody uptake.19,20 One of these studies used the zeolite nanocrystals functionalized with cetuximab antibodies in cancer cells that overexpressed the EGFR. The results revealed that the cellular uptake of cetuximab and zeolite nanocrystal bioconjugate over the targeted cancer cells was around 10-fold faster than that observed in the negative control cells.20
As one intriguing tactic, fibrosis pathway antagonists have attracted extensive attention for improving antibody uptake in solid tumors. These antagonists seek to reduce the amount of ECM that accumulates in tumor tissues, as this can obstruct the administration of antibodies. Relaxin-2, a peptide hormone, is one of these antagonists that has demonstrated promise in reducing fibrosis caused by transforming growth factor-beta by attaching to the relaxin family peptide receptor.21,22
In a study by Brown et al, second harmonic generation imaging was used to assess relaxin’s effect on tumor collagen. With HSTS26T xenografts implanted in dorsal skinfold chambers, the mice were subjected to a 12-day relaxin infusion by the researchers. The outcomes showed that relaxin infusion led to a decrease in the preexisting collagen fiber signal and the length of the tumor’s collagen fibers. Consequently, a non-specific immunoglobulin G antibody’s diffusion coefficient considerably increased by 80%, suggesting better antibody uptake and dispersion inside the tumor.23
Moreover, researchers employed genetically altered hematopoietic stem cells to investigate the possibility of the intra-tumoral production of relaxin. This method was evaluated on mice given xenografts of HCC1954 and BT474-M1. The results demonstrated that trastuzumab, an antibody used to treat some forms of breast cancer, performed better when there was relaxin expression in the tumor microenvironment.24 These findings indicate that relaxin, an antagonist of the fibrosis pathway, may improve the transport and uptake of therapeutic antibodies in solid tumors. Relaxin increases the accessibility of antibodies to tumor cells by shortening collagen fibers and altering the ECM, which may enhance the therapeutic benefits of antibodies.
Eikenes et al studied the effect of transcapillary pressure gradient on the transvascular and interstitial convection in solid tumors. They modulated the tumor ECM with ECM-degrading enzyme collagenase and assessed the interstitial fluid pressure changes in human osteosarcoma xenografts using the wick-in-needle and micropipette methods. They proved that collagenase could decrease interstitial fluid pressure (45%) and increase the transcapillary pressure gradient, thus inducing a 2-fold increase in the osteosarcoma-associated monoclonal antibody (TP-3) tumor uptake and improving its distribution.13
Using hyaluronidase to break down hyaluronan has been one of the studied methods for improving the uptake of co-administered mAbs. One ECM component called hyaluronan has the ability to act as a barrier, preventing therapeutic agents, including mAbs, from penetrating and spreading widely. The study findings demonstrated a noticeable enhancement in mAb uptake subsequent to hyaluronidase administration. In particular, there was a considerable 70% increase in the tumor cells’ uptake of the TP3 mAb following the intratumoral injection of bovine hyaluronidase.25
Another strategy to increase the uptake and efficacy of antibodies is to use combination therapies. For example, in a study in 2021, a combination of molecular and external radiotherapy was utilized in head and neck squamous cell carcinoma xenograft tumors, and it was concluded that a combination of radiolabeled cetuximab with clinically relevant fractionated radiotherapy has a significant potential to increase drug uptake.26
It is also possible to combine mAb immunotherapy and seven vasoactive immunoconjugates to significantly enhance antibody uptake in tumors and thereby increase the therapeutic index of immunotherapy. Vasoactive immunoconjugates optionally change the vascular permeability and blood volume of tumors. These immunoconjugates can be a combination of tumor necrosis factor-alpha, interleukin 2, interleukin 18, physalaemin, histamine, leukotriene B, and bradykinin chemically linked to tumor necrosis treatment-1, a murine mAb that attaches to necrotic regions in tumors.27
Another factor related to the amount of antibody uptake is the level of antigen expression. Studies established that, under saturating conditions, the uptake of antibodies is dependent on the number of available binding sites. Furthermore, small micrometastasis targeting is revealed to be higher than larger vascularized tumors. These results are consistent with those of previous research, indicating that the uptake of high-affinity antibodies relies on antigen expression levels for saturating doses (Figure 1).10
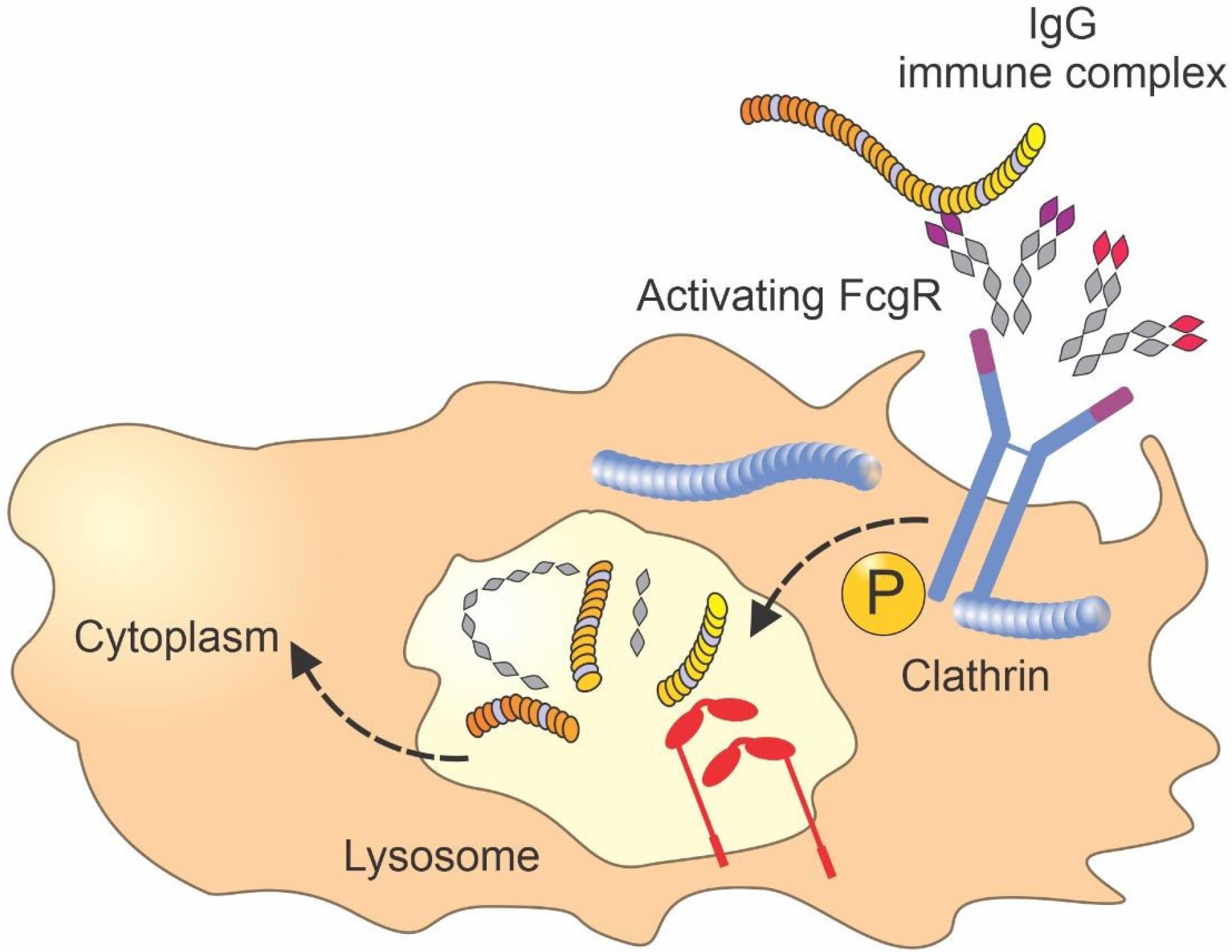
Figure 1.
Fcγ Receptors and Their Role in Antibody Uptake. Note. Fc: Fragment crystallizable; IgG: Immunoglobulin G
.
Fcγ Receptors and Their Role in Antibody Uptake. Note. Fc: Fragment crystallizable; IgG: Immunoglobulin G
Finally, there are also some factors that are inversely related to antibody uptake; for example, radioimmunotherapy is inversely associated with the size and location of the tumor within the host organ and is further effective against solid tumor micrometastases. Therefore, small metastatic deposits had higher uptake and prolonged retention at later time points.14
Conclusion
Antibody therapies have become one of the most significant anti-cancer therapeutics and have been used for the treatment of neurodegenerative disorders due to their remarkable progress over the last decade. In this study, it was revealed that the effectiveness of antibodies, especially mAb therapies, in solid tumors is restricted because of elements that impede the localization and uptake of antibodies, such as the ECM and abnormal vasculature of tumors. In this review, we summarized several approaches that have been investigated to enhance the uptake of antibodies. These strategies include the use of MR-guided FUS, which temporarily disrupts the BBB and enhances antibody delivery to HGG and the development of novel bivalent brain shuttles to overcome the BBB in AD by recombinantly attaching two single-chain variable fragments of the transferrin receptor antibody to the C-terminal end of mAb158, leading to an improvement in mAb uptake. Cholesterol-sequestering agents and nanomaterials, such as zeolite nanocrystals that enhanced the cellular uptake of cetuximab, have also been utilized to improve the uptake of anti-EGFR antibodies in carcinoma cells. Another strategy is to utilize a number of fibrosis pathway antagonists, most notably relaxin-2, which has demonstrated promise in solid tumors for both fibrosis reduction and improved antibody transport and uptake. Even when hematopoietic stem cells were genetically altered to express relaxin in mice that were given xenografts of HCC1954 and BT474-M1, trastuzumab worked more effectively. Additionally, the collagenase-mediated modification of the tumor ECM and the hyaluronidase-mediated breakdown of hyaluronan barriers have been confirmed to enhance antibody uptake. The use of combination therapies to boost antibody absorption and efficacy has undergone investigation. Examples of such combinations include molecular and external radiotherapy or mAb immunotherapy and vasoactive immunoconjugates.
In summary, this research has elucidated various tactics and variables that may impact the uptake of antibodies in distinct therapeutic settings, offering perspectives on plausible methods to augment the effectiveness of antibody-based interventions.
Authors’ Contribution
Conceptualization: Shiva Alipour, Leili Aghebati-Maleki.
Data curation: Shiva Alipour.
Formal analysis: Shiva Alipour.
Investigation: Shiva Alipour.
Methodology: Shiva Alipour.
Project administration: Leili Aghebati-Maleki.
Resources: Shiva Alipour.
Software: Shiva Alipour.
Supervision: Leili Aghebati-Maleki.
Validation: Leili Aghebati-Maleki.
Visualization: Rozita Abolhasan.
Writing–original draft: Shiva Alipour.
Writing–review & editing: Shiva Alipour, Leili Aghebati-Maleki.
Competing Interests
The authors declare that they do not have any conflict of interest.
Ethical Approval
Not applicable.
References
- Dahlén E, Veitonmäki N, Norlén P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother 2018; 6(1):3-17. doi: 10.1177/2515135518763280 [Crossref] [ Google Scholar]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359(6382):1350-5. doi: 10.1126/science.aar4060 [Crossref] [ Google Scholar]
- Montoliu-Gaya L, Villegas S. Immunotherapy for neurodegenerative diseases: the Alzheimer’s disease paradigm. Curr Opin Chem Eng 2018; 19:59-67. doi: 10.1016/j.coche.2017.12.006 [Crossref] [ Google Scholar]
- Xenaki KT, Oliveira S, van Bergen En Henegouwen PM. Antibody or antibody fragments: implications for molecular imaging and targeted therapy of solid tumors. Front Immunol 2017; 8:1287. doi: 10.3389/fimmu.2017.01287 [Crossref] [ Google Scholar]
- Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med 2014; 11(1):20-33. doi: 10.7497/j.issn.2095-3941.2014.01.002 [Crossref] [ Google Scholar]
- Gül N, van Egmond M. Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res 2015; 75(23):5008-13. doi: 10.1158/0008-5472.Can-15-1330 [Crossref] [ Google Scholar]
- Thurber GM, Dane Wittrup K. A mechanistic compartmental model for total antibody uptake in tumors. J Theor Biol 2012; 314:57-68. doi: 10.1016/j.jtbi.2012.08.034 [Crossref] [ Google Scholar]
- Bordeau BM, Balthasar JP. Strategies to enhance monoclonal antibody uptake and distribution in solid tumors. Cancer Biol Med 2021; 18(3):649-64. doi: 10.20892/j.issn.2095-3941.2020.0704 [Crossref] [ Google Scholar]
- Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM. Influence of affinity and antigen internalization on the uptake and penetration of anti-HER2 antibodies in solid tumors. Cancer Res 2011; 71(6):2250-9. doi: 10.1158/0008-5472.Can-10-2277 [Crossref] [ Google Scholar]
- Thurber GM, Weissleder R. Quantitating antibody uptake in vivo: conditional dependence on antigen expression levels. Mol Imaging Biol 2011; 13(4):623-32. doi: 10.1007/s11307-010-0397-7 [Crossref] [ Google Scholar]
- Bien-Ly N, Yu YJ, Bumbaca D, Elstrott J, Boswell CA, Zhang Y. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J Exp Med 2014; 211(2):233-44. doi: 10.1084/jem.20131660 [Crossref] [ Google Scholar]
- Sands H, Jones PL, Shah SA, Palme D, Vessella RL, Gallagher BM. Correlation of vascular permeability and blood flow with monoclonal antibody uptake by human Clouser and renal cell xenografts. Cancer Res 1988; 48(1):188-93. [ Google Scholar]
- Eikenes L, Bruland Ø S, Brekken C, Davies Cde L. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res 2004; 64(14):4768-73. doi: 10.1158/0008-5472.Can-03-1472 [Crossref] [ Google Scholar]
- Dearling JL, Flynn AA, Qureshi U, Whiting S, Boxer GM, Green A. Localization of radiolabeled anti-CEA antibody in subcutaneous and intrahepatic colorectal xenografts: influence of tumor size and location within host organ on antibody uptake. Nucl Med Biol 2009; 36(8):883-94. doi: 10.1016/j.nucmedbio.2009.07.003 [Crossref] [ Google Scholar]
- Hauck ML, Coffin DO, Dodge RK, Dewhirst MW, Mitchell JB, Zalutsky MR. A local hyperthermia treatment which enhances antibody uptake in a glioma xenograft model does not affect tumour interstitial fluid pressure. Int J Hyperthermia 1997; 13(3):307-16. doi: 10.3109/02656739709023538 [Crossref] [ Google Scholar]
- Brighi C, Reid L, White AL, Genovesi LA, Kojic M, Millar A. MR-guided focused ultrasound increases antibody delivery to nonenhancing high-grade glioma. Neurooncol Adv 2020; 2(1):vdaa030. doi: 10.1093/noajnl/vdaa030 [Crossref] [ Google Scholar]
- Hultqvist G, Syvänen S, Fang XT, Lannfelt L, Sehlin D. Bivalent brain shuttle increases antibody uptake by monovalent binding to the transferrin receptor. Theranostics 2017; 7(2):308-18. doi: 10.7150/thno.17155 [Crossref] [ Google Scholar]
- Chen Y, Liu G, Guo L, Wang H, Fu Y, Luo Y. Enhancement of tumor uptake and therapeutic efficacy of EGFR-targeted antibody cetuximab and antibody-drug conjugates by cholesterol sequestration. Int J Cancer 2015; 136(1):182-94. doi: 10.1002/ijc.28950 [Crossref] [ Google Scholar]
- Kapara A, Brunton V, Graham D, Faulds K. Investigation of cellular uptake mechanism of functionalised gold nanoparticles into breast cancer using SERS. Chem Sci 2020; 11(22):5819-29. doi: 10.1039/d0sc01255f [Crossref] [ Google Scholar]
- Marega R, Prasetyanto EA, Michiels C, De Cola L, Bonifazi D. Fast targeting and cancer cell uptake of luminescent antibody-nanozeolite bioconjugates. Small 2016; 12(39):5431-41. doi: 10.1002/smll.201601447 [Crossref] [ Google Scholar]
- Mookerjee I, Unemori EN, Du XJ, Tregear GW, Samuel CS. Relaxin modulates fibroblast function, collagen production, and matrix metalloproteinase-2 expression by cardiac fibroblasts. Ann N Y Acad Sci 2005; 1041:190-3. doi: 10.1196/annals.1282.028 [Crossref] [ Google Scholar]
- Mookerjee I, Hewitson TD, Halls ML, Summers RJ, Mathai ML, Bathgate RA. Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. FASEB J 2009; 23(4):1219-29. doi: 10.1096/fj.08-120857 [Crossref] [ Google Scholar]
- Brown E, McKee T, di Tomaso E, Pluen A, Seed B, Boucher Y. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med 2003; 9(6):796-800. doi: 10.1038/nm879 [Crossref] [ Google Scholar]
- Beyer I, Li Z, Persson J, Liu Y, van Rensburg R, Yumul R. Controlled extracellular matrix degradation in breast cancer tumors improves therapy by trastuzumab. Mol Ther 2011; 19(3):479-89. doi: 10.1038/mt.2010.256 [Crossref] [ Google Scholar]
- Brekken C, Hjelstuen MH, Bruland Ø S, de Lange Davies C. Hyaluronidase-induced periodic modulation of the interstitial fluid pressure increases selective antibody uptake in human osteosarcoma xenografts. Anticancer Res 2000; 20(5B):3513-9. [ Google Scholar]
- Dietrich A, Andreeff M, Koi L, Bergmann R, Schubert M, Schreiner L. Radiotherapy enhances uptake and efficacy of 90Y-cetuximab: a preclinical trial. Radiother Oncol 2021; 155:285-92. doi: 10.1016/j.radonc.2020.11.013 [Crossref] [ Google Scholar]
- Khawli LA, Miller GK, Epstein AL. Effect of seven new vasoactive immunoconjugates on the enhancement of monoclonal antibody uptake in tumors. Cancer 1994; 73(3 Suppl):824-31. doi: 10.1002/1097-0142(19940201)73:3+<824::aidcncr2820731312>3.0.co;2-v [Crossref] [ Google Scholar]