Biomed Res Bull. 1(2):53-59.
doi: 10.34172/biomedrb.2023.11
Original Article
Down-regulation of Toll-like Receptor 2 Via HMGB1 Blocking With Kaempferol on Kupffer Cells From Hepatocellular Carcinoma Patients
Zhang Yue 1  , Tian Ma 2, Xu Zhang 1, Tong Ma 2, *
, Tian Ma 2, Xu Zhang 1, Tong Ma 2, * 
Author information:
1Department of Immunology, Faculty of Medicine, Jiangsu University, Zhenjiang, China
2Department of Surgery, First Affiliated Hospital of Suzhou University, Suzhou, China
Abstract
Background:
Kaempferol is a pharmacological kaempferol that can inhibit many inflammatory factors implicated in cancer.
Methods:
The present study focused on kaempferol function on the immunity signaling pathway. In this study, Kupffer cells were also isolated with new methods.
Results:
Our results indicated that the levels of interleukin 17 (IL-17), IL-33, and IL-6 in supernatants from cultured Kupffer cells from patients were increased after stimulation with high mobility group box-1 (HMGB1), but the levels of these cytokines decreased after using kaempferol to prevent inflammation.
Conclusion:
The findings of this study imply that the reduction of the Toll-like receptor 2 (TLR2) pathway and T helper 17 cell polarization may be due to HMGB1 blocking with Kaempferol in hepatocellular carcinoma.
Keywords: Kaempferol, HMGB1, TLR2
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Kaempferol is a flavonoid widely distributed in nature. Flavonoids are polyphenolic compounds found in various foods of plant origin. It has been estimated that humans consuming high amounts of fruit and vegetable diets may invest up to 1 g of these compounds daily.1-3 Kaempferol has a broad range of activities within cells.4 It has anti-inflammatory effects, regulating nitric oxide, interleukin 6 (IL-6), and tumor necrosis factor-alpha release and inhibiting lipopolysaccharide (LPS)-induced delay in spontaneous apoptosis and activation of neutrophils.5 In tumor cells, it exerts anti-proliferative effects and arrests human leukemic T cells in the late G1 phase of the cell cycle.6
Kupffer cells, the resident macrophages of the liver, are poised to initiate innate immune responses through reciprocal interactions with local natural killer (NK) cells when primed by pathogen-derived products. Innate immune defenses can be similarly activated by neoplasms7 or even systemic inflammation.8 This particular activation of macrophages and NK cells and their tight reciprocal regulation occurs through the ligation of Toll-like receptors (TLRs).8,9 These receptors are innate immunity receptors that have an important role in inflammation and cancer.10 Furthermore, TLRs are able to recognize endogenous alarmins released by damaged tissues and necrosis and/or apoptotic cells and are present in numerous autoimmune diseases.11 Therefore, the release of endogenous TLR ligands plays an important role in initiating and driving inflammation12-15 In this way, TLRs may be crucial cellular sentinels to detect danger signals such as high mobility group box-1 (HMGB1) during inflammation. IL-33 is a recently identified member of the IL-1 family and has been shown to function as a ligand for the IL-1 receptor-like1s, also known as ST2, which is a member of the TLR domain-containing superfamily.15 In contrast to all of the well-characterized members of the TLR family that induce the inflammatory response via the activation of nuclear factor-kappa B (NF-κB), signaling through ST2 is unable to activate NF-kB although it can activate mitogen-activated protein kinase (MAPK).16
Hepatocellular carcinoma (HCC) is the third most frequent neoplasm worldwide.17 Its prevalence is peaking rapidly compared to other cancers possibly due to a high prevalence of hepatitis B and C viral infections. Recently some studies have reported that Kaempferol plays a role in anti-inflammation.18 It must be reasonable to speculate upon the inhibitory effect of Kaempferol on TLR-mediated MAPK activation and further inflammatory events. Thus, the present study focused on the effect of kaempferol on the mRNA expression of TLR2 and MAPK activation with the stimulation of HMGB1, cytokine production, activities of different inflammatory enzymes induced during inflammation in humans, and the effect of this kaempferol on HCC with the inhibition of inflammation.
Materials and Methods
Reagents
TLR2 primer was obtained from Chingdao Company, Nanjing-China. IL-6 and IL-33 enzyme-linked immunosorbent assay (ELISA) kits were purchased from Abcam-USA. The CD14 antibody was purchased from Invitrogen-USA.
Mice Model Preparation
Kaempferol was prepared for each experiment by dissolving kaempferol to a concentration of 30 mg/mL in dimethyl-sulfoxide warmed to 40 °C. DEN mice were fasted for 10 hours and then injected intraperitoneally with phosphate-buffered saline (PBS) or Kaempferol at 400 mg/kg (body weight). At the indicated time points, plasma was collected and stored at -20 °C for measuring total bilirubin and cytokines. The livers were excised, and portions were snap-frozen in liquid nitrogen, stored at -80 °C for RNA isolation, and then fixed in 4% paraformaldehyde for tissue sections stained with hematoxylin/eosin (H&E). Kaempferol was monitored for any impact on the pathogenesis of inflammation.
Patients
HCC and adjacent tissues were obtained from 20 patients at the time of surgical resection at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). Informed consent for patient sample analysis was obtained from each subject prior to surgery. Fibrotic liver tissues were collected following partial hepatectomy due to early-stage HCC which is based on cirrhosis; control livers are normal tissues obtained from hepatectomy for hepatic hemangioma.
Isolation and Culture of Kupffer Cells
Human Kupffer cells were isolated from normal liver tissues obtained from fresh surgical hepatectomy specimens with the assistance of the Jiangsu Province Hospital (Nanjing-China). Liver wedge biopsies were directly kept on ice after resection, and the isolation procedure was started within 10-15 minutes. Specimens were weighed and cut into small tissue fragments (1-2 mm3) in Gey’s balanced salt solution (GBSS). The liver homogenate was then incubated in 75 mL GBSS with 0.025 g collagenase type-1 on a magnetic stirrer at 37 °C with continuous pH registration. After 10 minutes, the suspension and the rest of the fragments were filtered through a gauze (pore 60 pm). The suspension was centrifuged at 100 g for 5 minutes and washed twice by resuspending the pellet in 50 mL GBSS and 0.8 pg/mL DNase. After washing, the pellet was resuspended in 5 mL GBSS and 0.8 pg/mL DNase. The non-parenchymal cells were separated from nonviable cells, the remaining parenchymal cells, and erythrocytes by centrifugation on a 16% Nycodenz (Nycomed AS, Oslo, Norway) gradient for 20 minutes at 300 g at 4 °C. The low-density fraction was collected, resuspended in 10 mL GBSS, and 0.8 pg/mL DNase, and centrifuged at 100 g for 5 minutes. The final pellet was resuspended in 5 mL GBSS and 0.8 pg/mL DNase. Cells (4 × 106 cells/well in a 6-well plate) were plated in tissue culture plates at 37 °C for one-half hour before washing and incubating in tissue culture media containing 5% FBS overnight. These cells were 53% pure for Kupffer cells as estimated by their ability to ingest latex beads (Figure 1A-E). Cell viability was always 90% as assessed by trypan blue. All experiments were subsequently performed after washing the cells three times with serum-free media. For each experiment, Kupffer cells were isolated from one individual liver. The amount of the applied liver was variable and relied on the amount of excess liver tissue available from each surgical operation. The Kupffer cells from the normal liver had a long time to be active, while those from the HCC liver were activated; then, we were unable to keep the cells for a long time.
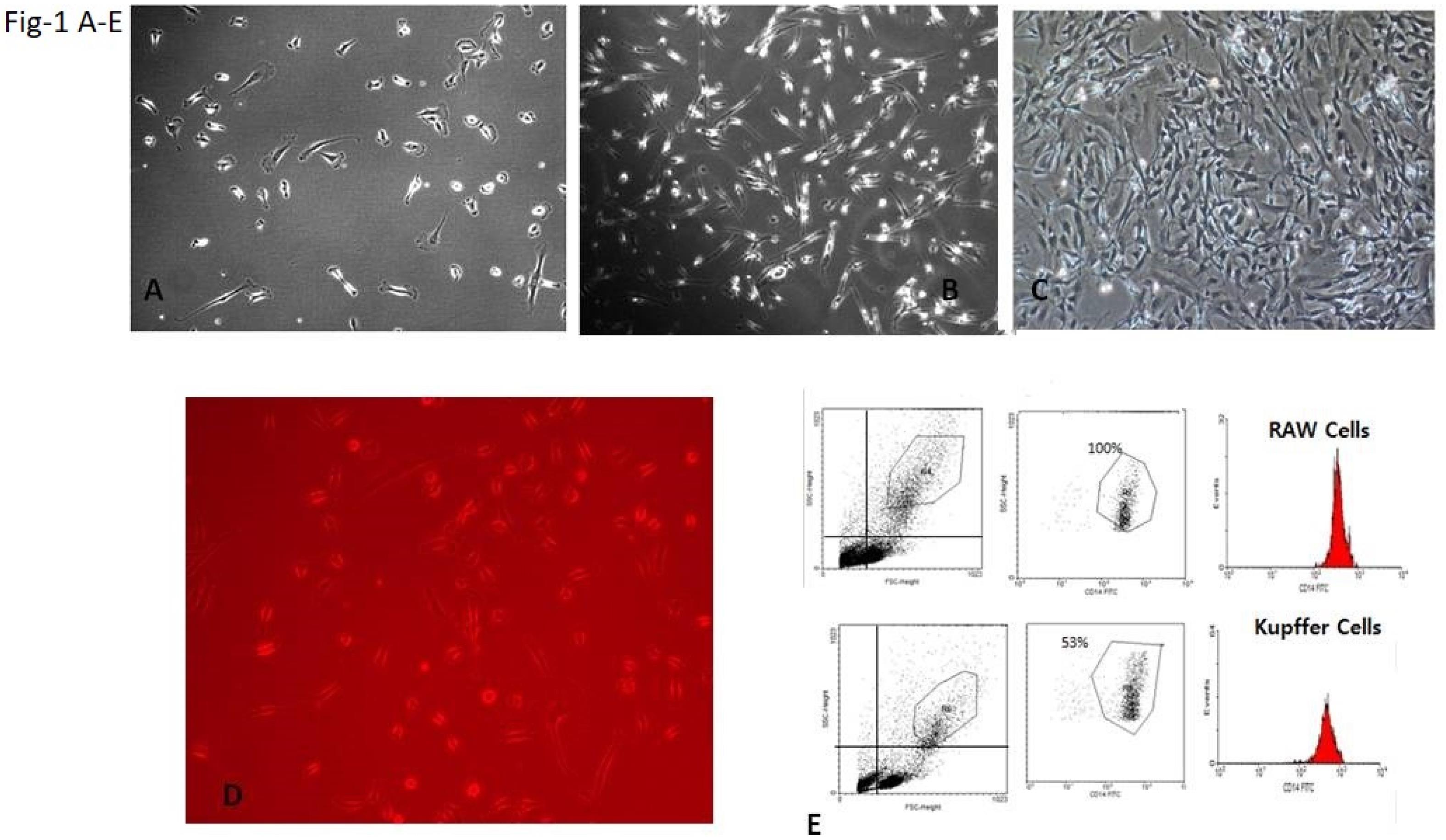
Figure 1.
Kupffer Cell Isolation: Kupffer Cells After (A) 10 Minutes of Isolation, (B) One Week of Isolation, (C) Two Weeks of Isolation, (D) Phagocytosis Function After One Week With Immune Fluorescence Microscope, and (E) The Result of Counting Kupffer Cells After Isolation and Comparison With RAW Cell Lines as a Control. Note. The analysis showed that 53% of cells are live cells.
.
Kupffer Cell Isolation: Kupffer Cells After (A) 10 Minutes of Isolation, (B) One Week of Isolation, (C) Two Weeks of Isolation, (D) Phagocytosis Function After One Week With Immune Fluorescence Microscope, and (E) The Result of Counting Kupffer Cells After Isolation and Comparison With RAW Cell Lines as a Control. Note. The analysis showed that 53% of cells are live cells.
Cell Culture and High Mobility Group Box-1 Stimulation
Kupffer cells isolated from HCC patients and normal tissues were cultured and treated with HMGB1 with time courses 6, 12, 24, 48, and 72 hours. Anti-human TLR2 antibody was used to block the TLR2 before HMGB1 treatment. Then, 2 mg/mL kaempferol was dissolved in ethanol and 0.5% of the total volume of cell culture was added before treatment with HMGB1.
Histopathology
Livers from patients were fixed in 10% formalin, paraffin embedded, and stained with H&E. Severity scores of the liver were graded in a double-blind manner by two independent investigators according to the Dallas criteria based on the presence of inflammatory cell infiltration.
Reverse Transcriptase-polymerase Chain Reaction Analysis for Toll-like Receptor 2
The total RNA of the Kupffer cells was extracted with TRIzol reagent (Invitrogen) and different kinds of mRNA in the liver using specific primers (Table 1; Invitrogen Shanghai Company Ltd). PCR products were analyzed by electrophoresis on a 1.5% agarose gel. The real-time PCR was used for quantity analysis of TLR2 expression, and B-actin was employed as a control.
Table 1.
Primer sequences
|
Primers
|
Sequence
|
TLR2 F
TLR2 R |
3' AAGGTTAGATCCCTTAAAAGTA5'
3' GGTTAAGGTCCTTACCGGAATTC5' |
| B-actin |
3' GGAATTGGACCCCTTAACCATGG5'
3' TTAAGGGTTTCCCAGGTTAGGCTA5' |
Note. TLR: Toll-like receptor 2.
Flow Cytometry Analysis
Kupfer cells were isolated from the liver and treated with kaempferol, and the cells were incubated with anti-CD14 mAb for measuring the amount of Kupfer cells. After washing, the cells were re-suspended in 1% FCS/PBS. Analysis was performed on a flow cytometer (Becton Dickinson, USA). RAW cell lines were chosen as a standard group.
Immunofluorescence
Immunofluorescence staining of paraffin-embedded Kupffer cells from HCC patients was performed as described previously.19 After deparaffinization, rehydration, and antigen unmasking, the samples were immersed in a blocking buffer for 60 minutes; then, primary antibodies, namely, anti-CD4 and anti-IL-17 (Santa Cruz Biotechnology, USA), were applied for 2 hours at room temperature. After washing, FITC- and PE-labeled secondary antibodies were added for 1 hour. The Kupffer cells from HCC patients and healthy groups were stimulated with HMGB1 for 12 hours and then incubated with anti-human IL-17 antibody for 2 hours at room temperature. After washing, the PE-labeled secondary antibody (PE-labeled anti-human immunoglobulin G) was added and re-incubated for another 1 hour. The sections were viewed with a fluorescence microscope (Olympus, Japan) and analyzed using the ImageJ software.
Cytokine Measurements
Cell supernatant was collected and stored at -80 °C until use. IL-1b, IL-6, IL-17, and IL33 were measured using ELISA kits (Bender MedSystems, Austria) according to the manufacturer’s instructions.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism 5 software. Data were expressed as the mean ± standard deviation (SD) in the text and figures.
Results
Liver Protection by Kaempferol in Hepatocellular Carcinoma
H&E staining indicated that kaempferol resulted in an extremely reduced infiltration of inflammatory cells in the liver (Figure 2A). The gross severity score of the kaempferol-treated group was significantly lower than that of untreated DEN mice (4.50370.82 versus 2.0070.67, P 0.001; Figure 2B). Treatment with kaempferol markedly reduced the collagen percentage of Sirius-red-stained areas in the DEN mice. Overall, the results demonstrated that kaempferol has a protective effect on liver inflammation in HCC.
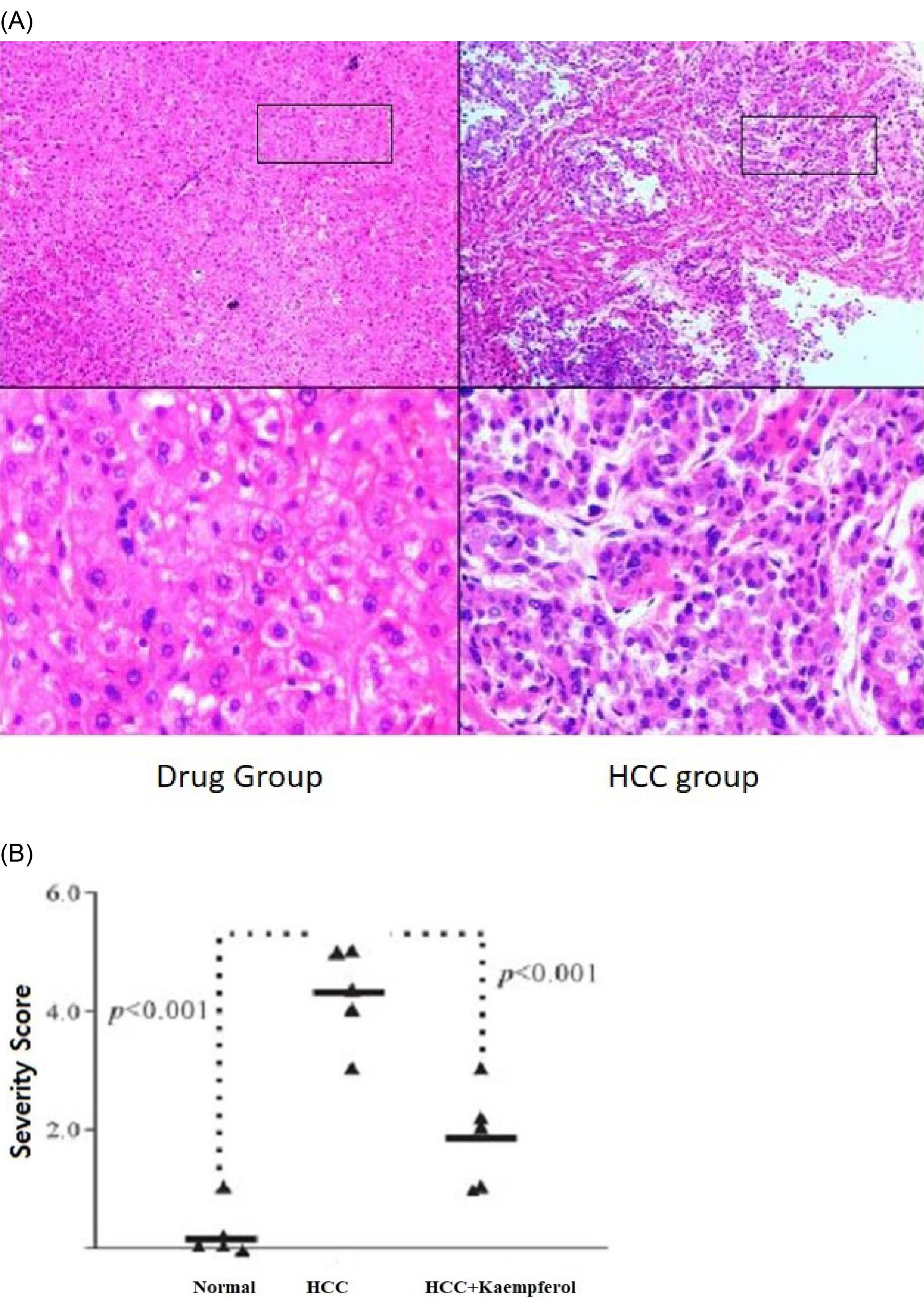
Figure 2.
(A) H&E Staining and (B) the Pathology Scored for Severity. Note. H&E: Hematoxylin and eosin. On day 21, the liver sections were stained with H&E. A representative image is shown for each group. Each triangle demonstrates a mouse. The horizontal bar denotes the mean.
.
(A) H&E Staining and (B) the Pathology Scored for Severity. Note. H&E: Hematoxylin and eosin. On day 21, the liver sections were stained with H&E. A representative image is shown for each group. Each triangle demonstrates a mouse. The horizontal bar denotes the mean.
Increased Frequency of T Helper 17 Cells in Kupffer Cells From Patients With Hepatocellular Carcinoma Stimulated by High Mobility Group Box-1
The results of immunofluorescent staining showed that the number of Th17 cells from patients with HCC was enhanced after HMGB1 stimulation in vitro compared with the normal group (P < 0.001, Figure 3). Further, our data demonstrated the same result in tissue samples from the patient and normal groups.
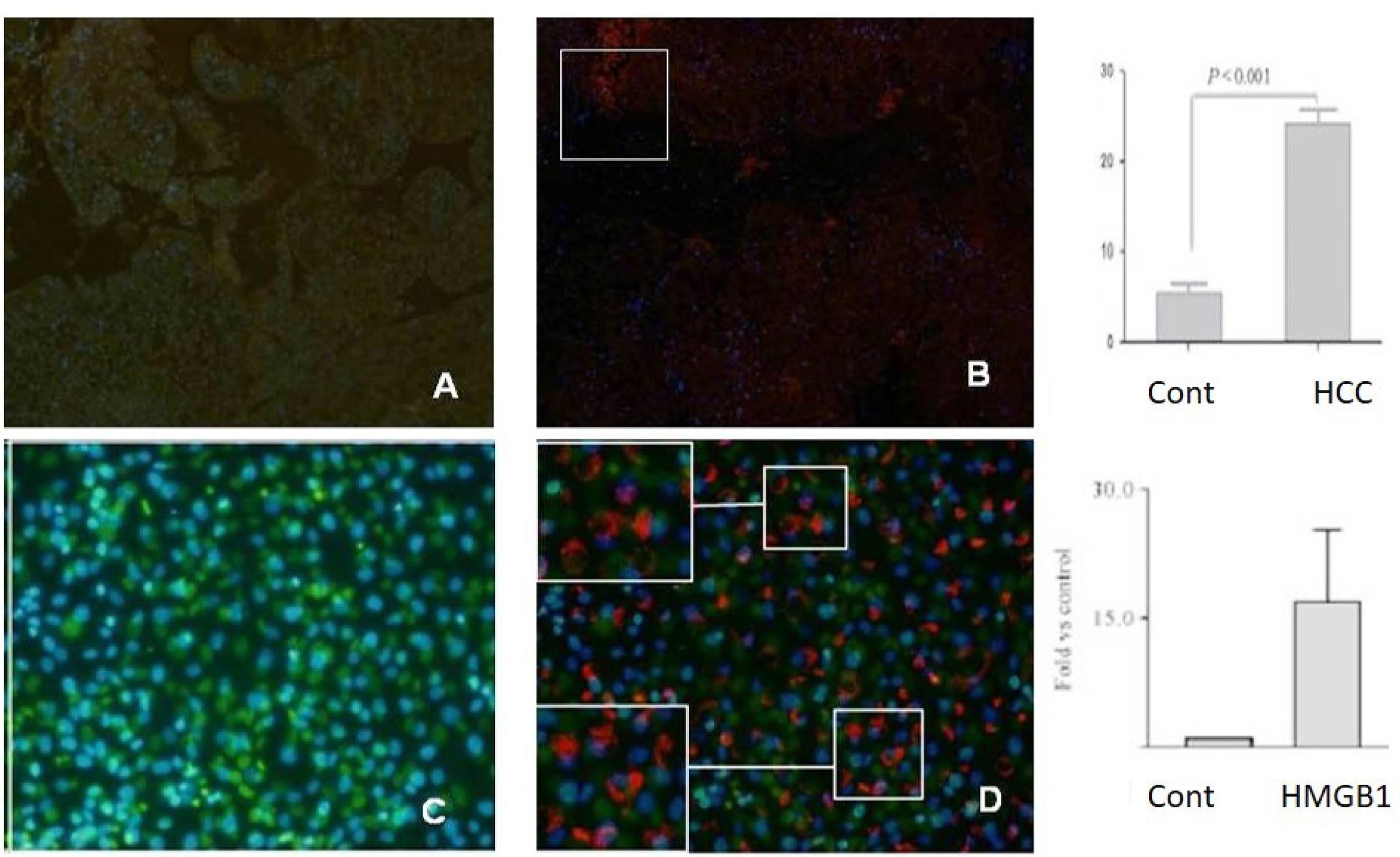
Figure 3.
The Results of Immunofluorescent Staining. Note. HCC: Hepatocellular carcinoma; Th17: T helper 17. The images in the upper half indicate the results of observation on immunofluorescent staining by fluorescence microscopy; the red section represents positive staining cells (Th17 cells), and increased numbers could be found in CD4 + T cells from HCC tissue patients, including (A) normal tissue and (B) HCC tissue. In addition, cells from unstimulated (C) and stimulated (D) stained with the same antibodies showed the same results. The histogram of lower half demonstrated that the frequency of the Th17 cell in CD4 + T cells from the Kupffer cells of patients with HCC was increased (P < 0.001) compared with the normal group.
.
The Results of Immunofluorescent Staining. Note. HCC: Hepatocellular carcinoma; Th17: T helper 17. The images in the upper half indicate the results of observation on immunofluorescent staining by fluorescence microscopy; the red section represents positive staining cells (Th17 cells), and increased numbers could be found in CD4 + T cells from HCC tissue patients, including (A) normal tissue and (B) HCC tissue. In addition, cells from unstimulated (C) and stimulated (D) stained with the same antibodies showed the same results. The histogram of lower half demonstrated that the frequency of the Th17 cell in CD4 + T cells from the Kupffer cells of patients with HCC was increased (P < 0.001) compared with the normal group.
Kaempferol Decreases Toll-like Receptor 2 Expression With Blocking High Mobility Group Box-1 In Vitro
The Kupffer cells were cultured and stimulated with HMGB1, and then mRNA levels of TLR2 were measured by qRT-PCR. As shown in Figure 4, the mRNA of TLR2 was increased in Kupffer cells with HMGB1 stimulation from patients and normal groups, especially when the cells were co-cultured with HMGB1 for 8 hours. Compared with previous results, TLR2 did not increase in the Kaempferol-treated group (data were compared with LPS).
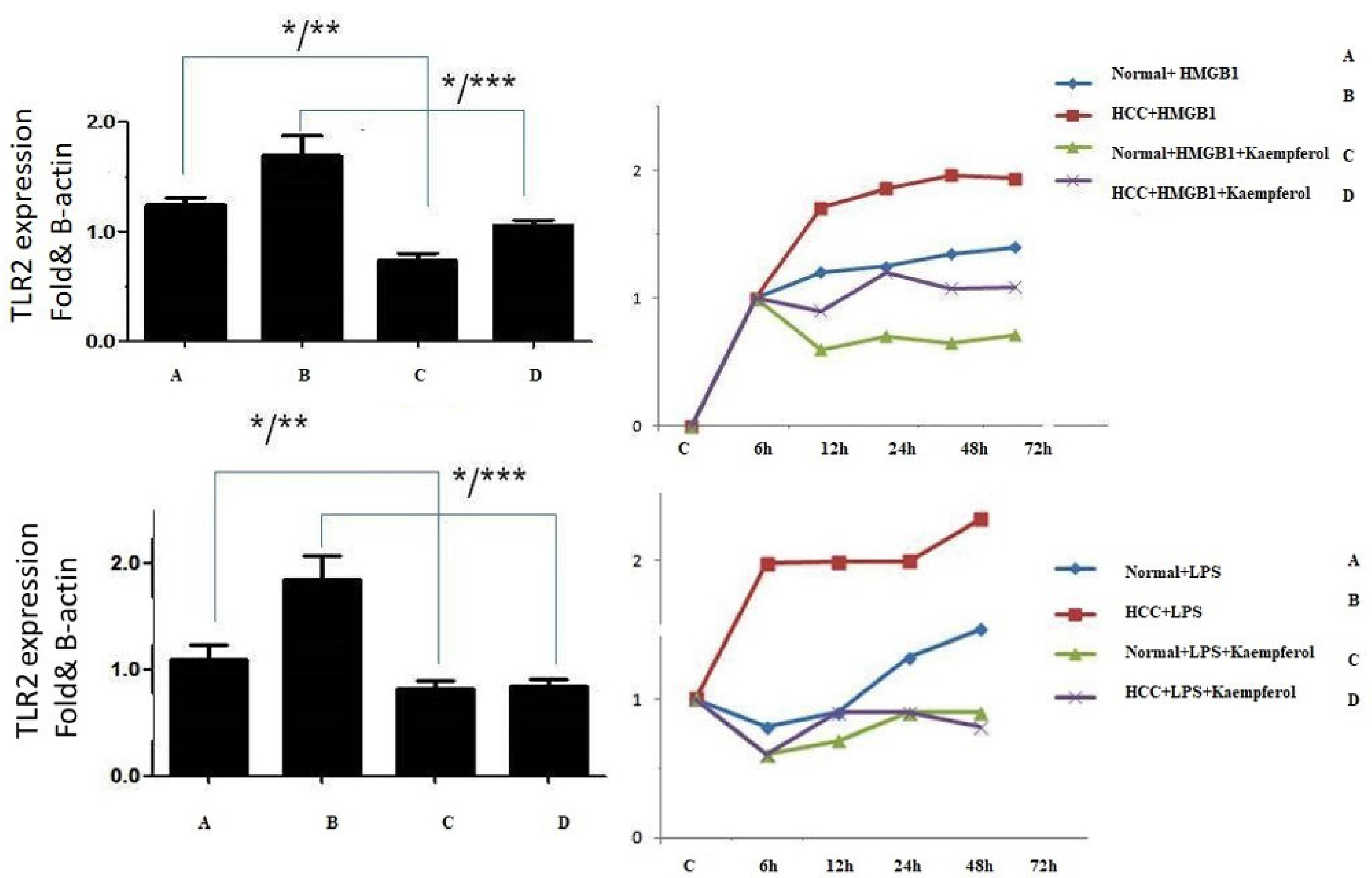
Figure 4.
TLR2 Expression on Human Kupffer Cells. Note. TLR2: Toll-like receptor 2; HMGB1: High mobility group box-1; LPS: Lipopolysaccharide. The upper left diagram illustrates the kaempferol effect on TLR2 expression on kupffer cell stimulation with HMGB1 and Kaempferol. The data represented in kaempferol group TLR2 can significantly decrease compared to the HMGB1 group (P < 0.001). In addition, the results compared with LPS showed the same data. Further, upper right diagram displayed in the time course TLR2 expression was high after 8 hours of stimulation.
.
TLR2 Expression on Human Kupffer Cells. Note. TLR2: Toll-like receptor 2; HMGB1: High mobility group box-1; LPS: Lipopolysaccharide. The upper left diagram illustrates the kaempferol effect on TLR2 expression on kupffer cell stimulation with HMGB1 and Kaempferol. The data represented in kaempferol group TLR2 can significantly decrease compared to the HMGB1 group (P < 0.001). In addition, the results compared with LPS showed the same data. Further, upper right diagram displayed in the time course TLR2 expression was high after 8 hours of stimulation.
Kaempferol Decrease Inflammation via Down-regulation of T Helper 17 Production by Kupffer Cells With the Inhibition of IL-6 and IL-33
Some studies reported that IL-33 can be produced by macrophages, but little is known about its transcriptional regulation by Kaempferol. Therefore, we initially measured IL-33 cytokine levels in mouse primary Kupffer cells after treatment with Kaempferol. As depicted in Figure 5A, Kupffer cell stimulation by Kaempferol strongly decreased IL-33 cytokine levels in a time-dependent manner. ELISA data revealed that Kaempferol can modulate proinflammatory cytokine (IL-6) production on Kupffer cells with the blockage of HMHB1 (Figure 5). To illustrate the effect of Kaempferol on HMGB1, Kaempferol (10 µM/mL) was added into the system of Kupffer cell culture 1 hour before treatment with HMGB1, and the cell supernatant was collected for ELISA. The data showed that Kaempferol could significantly decrease the expressions of IL-17 (Figure 5). To represent the specificity of HMGB1 function, LPS was compared with the HMGB1 effect on stimulating IL-17 secretion on Kupffer cells from healthy volunteers. TLR2 or HMGB1 blockade affected IL-17 secretion in the HMGB1 stimulation group, but it did not significantly affect IL-17 secretion in LPS stimulation groups (Figure 5).
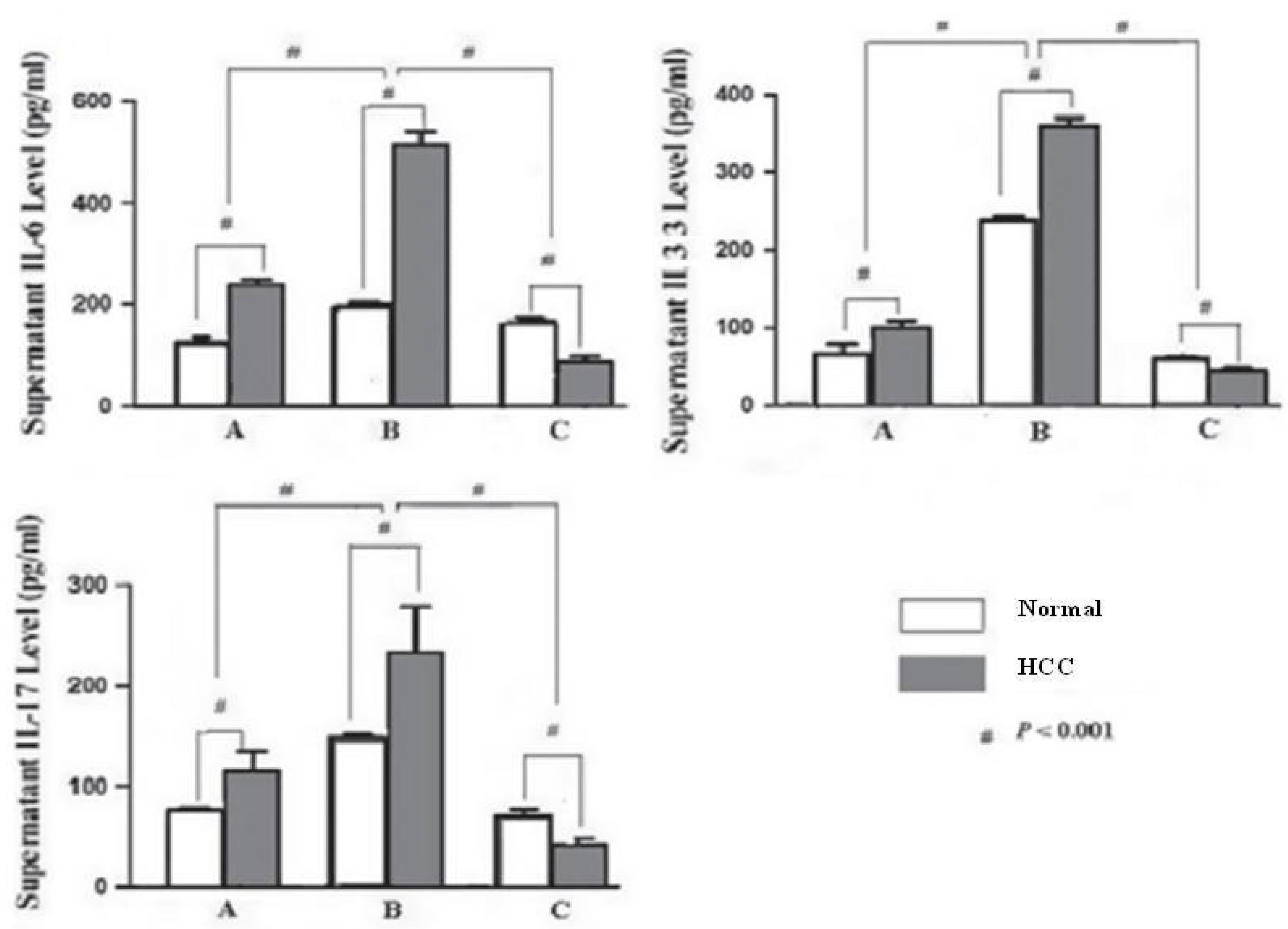
Figure 5.
The Levels of IL-6, IL-33, and IL-17 in the Supernatant. Note. IL: Interleukin
.
The Levels of IL-6, IL-33, and IL-17 in the Supernatant. Note. IL: Interleukin
Discussion
HCC, the most common type of human liver cancer, is the third leading cause of cancer death worldwide.20 In some African and Asian countries, HCC has become the most prevalent cause of cancer-related death.21 It usually develops in association with hepatitis B virus (HBV) and hepatitis C virus (HCV) infection and other liver diseases such as liver steatosis and fibrosis.22 The spread of HBV and HCV has resulted in approximately 500 million people with persistent virus infections,23 some of which will lead to liver cancer, causing a strong rise in HCC incidence. At the same time, the eradication of these chronic viral infections has turned out to be highly ineffective. Therefore, we went on to analyze and identify fundamental inflammatory signaling pathways causing the transition from liver injury to HCC and to treat patients with the inhibition of liver inflammation. Kupffer cells as macrophage cells are the most important proinflammatory cells in the host defense mechanism, including normal metabolism, phagocytosis, cytokine generation, and anti-tumor effects.24 They are also involved in the pathogenesis of liver diseases such as viral hepatitis, alcoholic liver injury, chemically mediated liver injury, liver fibrosis, ischemia and reperfusion injury in liver transplantation, and hepatocyte regeneration.25 Thus, we wanted to find a way to activate the cells, and then stop the signaling pathways and prevent cancer. Therefore, we stimulated Kupffer cells with HMGB1, which is a DNA-binding protein that can also be released extracellularly and functions as a late mediator of inflammatory responses. Although the results indicated that the TLR2 can be a good receptor for HMGB1 on Kupffer cells (Figure 6). Some studies discussed that TLR2 can be involved in ST2 and IL-33 activation.26 IL-33 mediates its biological effects by interacting with the receptors ST2 and IL-1 receptor accessory protein, activating intracellular molecules in the NF-κB and MAP kinase signaling pathways that drive the production of type 2 cytokines from polarized Th2 cells. The induction of type 2 cytokines by IL-33 in vivo is believed to induce severe pathological changes observed in mucosal organs following the administration of IL-33.27,28 Flavonoids have been recognized to exert antibacterial and antiviral activity, anti-inflammatory, anti-angiogenic, and anti-allergic effects, as well as analgesic, hepatoprotective, cytostatic, apoptotic, estrogenic, and anti-estrogenic properties.29,30 Figure 6 shows that Kaempferol can decrease proinflammatory cytokines such as IL-6 and IL-1β by blocking HMGB1. In conclusion, Kaempferol can decrease liver injury during HCC with a decrease in proinflammatory cytokines and IL-17 production.
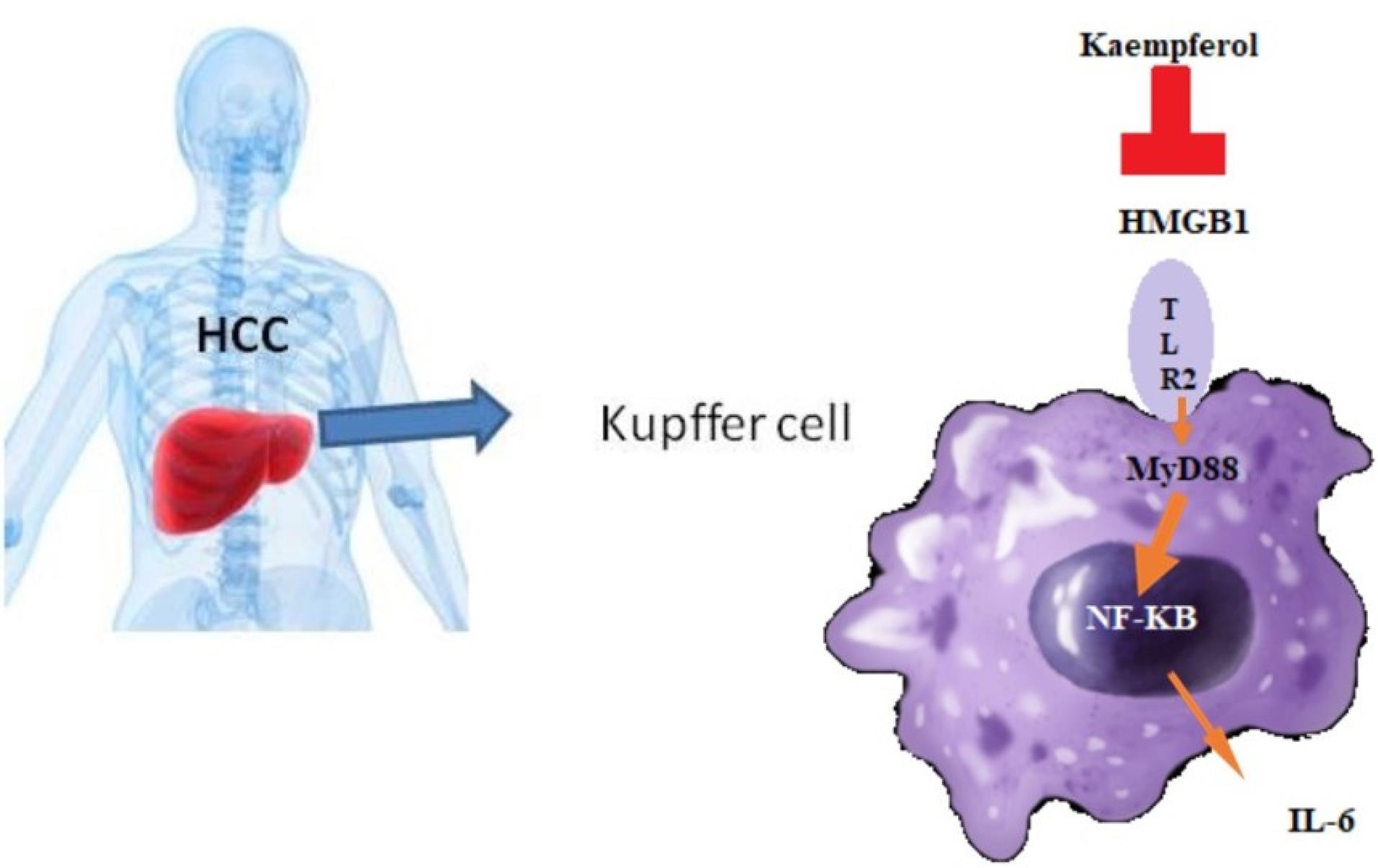
Figure 6.
The Diagram of the TLR2 Pathway Activated by HMGB1 in HCC and Effects of Kaempferol. Note. IL: Interleukin; TLR2: Toll-like receptor 2; HMGB1: High mobility group box-1; HCC: Hepatocellular carcinoma; Th17: T helper 17. Increased HMGB1 might stimulate Kupffer cells to secrete some Th17 cell-associated cytokines such as IL-6, IL-33, and the like and result in Th17 cell differentiation. Kaempferol as a downstream target of TLR2 also markedly decreased the HMGB1, which may be due to a decrease in IL-17 produced by Th17 cells.
.
The Diagram of the TLR2 Pathway Activated by HMGB1 in HCC and Effects of Kaempferol. Note. IL: Interleukin; TLR2: Toll-like receptor 2; HMGB1: High mobility group box-1; HCC: Hepatocellular carcinoma; Th17: T helper 17. Increased HMGB1 might stimulate Kupffer cells to secrete some Th17 cell-associated cytokines such as IL-6, IL-33, and the like and result in Th17 cell differentiation. Kaempferol as a downstream target of TLR2 also markedly decreased the HMGB1, which may be due to a decrease in IL-17 produced by Th17 cells.
Acknowledgments
The authors thank Beicheng Sun for his helpful discussions and comments.
Authors’ Contribution
Data curation: Zhang Yue.
Formal analysis: Tang Ma.
Funding acquisition: Zhang Yue.
Investigation: Tian Ma.
Methodology: Xu Zhang.
Project administration: Xu Zhang.
Software: Zhang Yue.
Supervision: Tang Ma.
Writing–original draft: Zhang Yue.
Competing Interests
None.
Ethical Approval
The study was approved by the Institutional Ethics Committee of China (Ethics No. 81072453).
Funding
This work was supported by the National Natural Science Foundation of China (81072453, 30972748, 81001319).
References
- Mojzisová G, Mirossay L, Kucerová D, Kyselovic J, Mirossay A, Mojzis J. Protective effect of selected flavonoids on in vitro daunorubicin-induced cardiotoxicity. Phytother Res 2006; 20(2):110-4. doi: 10.1002/ptr.1811 [Crossref] [ Google Scholar]
- Kinoshita T, Lepp Z, Chuman H. Construction of a novel database for flavonoids. J Med Invest 2005; 52 Suppl:291-2. doi: 10.2152/jmi.52.291 [Crossref] [ Google Scholar]
- Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr 1999; 38(3):133-42. doi: 10.1007/s003940050054 [Crossref] [ Google Scholar]
- Abdallah FB, Fetoui H, Fakhfakh F, Keskes L. Caffeic acid and quercetin protect erythrocytes against the oxidative stress and the genotoxic effects of lambda-cyhalothrin in vitro. Hum Exp Toxicol 2012; 31(1):92-100. doi: 10.1177/0960327111424303 [Crossref] [ Google Scholar]
- Yoshida M, Yamamoto M, Nikaido T. Kaempferol arrests human leukemic T-cells in late G1 phase of the cell cycle. Hum Exp Toxicol 2010; 18(1):80-90. [ Google Scholar]
- Heeba GH, Mahmoud ME, El Hanafy AA. Anti-inflammatory potential of curcumin and quercetin in rats: role of oxidative stress, heme oxygenase-1 and TNF-α. Toxicol Ind Health 2014; 30(6):551-60. doi: 10.1177/0748233712462444 [Crossref] [ Google Scholar]
- Yang Q, Shi Y, He J, Chen Z. The evolving story of macrophages in acute liver failure. Immunol Lett 2012; 147(1-2):1-9. doi: 10.1016/j.imlet.2012.07.002 [Crossref] [ Google Scholar]
- Kim SH, Serezani CH, Okunishi K, Zaslona Z, Aronoff DM, Peters-Golden M. Distinct protein kinase A anchoring proteins direct prostaglandin E2 modulation of toll-like receptor signaling in alveolar macrophages. J Biol Chem 2011; 286(11):8875-83. doi: 10.1074/jbc.M110.187815 [Crossref] [ Google Scholar]
- Sorrentino R, Morello S, Chen S, Bonavita E, Pinto A. The activation of liver X receptors inhibits toll-like receptor-9-induced foam cell formation. J Cell Physiol 2010; 223(1):158-67. doi: 10.1002/jcp.22022 [Crossref] [ Google Scholar]
- Feng Y, Chao W. Toll-like receptors and myocardial inflammation. Int J Inflam 2011; 2011:170352. doi: 10.4061/2011/170352 [Crossref] [ Google Scholar]
- Sandoghchian Shotorbani S, Su ZL, Xu HX. Toll-like receptors are potential therapeutic targets in rheumatoid arthritis. World J Biol Chem 2011; 2(7):167-72. doi: 10.4331/wjbc.v2.i7.167 [Crossref] [ Google Scholar]
- Sandoghchian Shotorbani S, Zhang Y, Baidoo SE, Xu H, Ahmadi M. IL-4 can inhibit IL-17 production in collagen induced arthritis. Iran J Immunol 2011; 8(4):209-17. [ Google Scholar]
- He Z, Sandoghchian Shotorbani S, Jiao Z, Su Z, Tong J, Liu Y. HMGB1 promotes the differentiation of Th17 via up-regulating TLR2 and IL-23 of CD14 + monocytes from patients with rheumatoid arthritis. Scand J Immunol 2012; 76(5):483-90. doi: 10.1111/j.1365-3083.2012.02759.x [Crossref] [ Google Scholar]
- Lu Z, Zhang X, Li Y, Jin J, Huang Y. TLR4 antagonist reduces early-stage atherosclerosis in diabetic apolipoprotein E-deficient mice. J Endocrinol 2013; 216(1):61-71. doi: 10.1530/joe-12-0338 [Crossref] [ Google Scholar]
- Zhang L, Lu R, Zhao G, Pflugfelder SC, Li DQ. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium. Int J Biochem Cell Biol 2011; 43(9):1383-91. doi: 10.1016/j.biocel.2011.06.003 [Crossref] [ Google Scholar]
- Yndestad A, Marshall AK, Hodgkinson JD, Tham EL, Sugden PH, Clerk A. Modulation of interleukin signalling and gene expression in cardiac myocytes by endothelin-1. Int J Biochem Cell Biol 2010; 42(2):263-72. doi: 10.1016/j.biocel.2009.10.021 [Crossref] [ Google Scholar]
- Fehér J, Lengyel G. [Hepatocellular carcinoma: occurrence, risk factors, biomarkers]. Orv Hetil 2010; 151(23):933-40. doi: 10.1556/oh.2010.28900.[Hungarian] [Crossref] [ Google Scholar]
- Angeloni C, Hrelia S. Quercetin reduces inflammatory responses in LPS-stimulated cardiomyoblasts. Oxid Med Cell Longev 2012; 2012:837104. doi: 10.1155/2012/837104 [Crossref] [ Google Scholar]
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007; 117(6):1538-49. doi: 10.1172/jci30634 [Crossref] [ Google Scholar]
- Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol 2012; 40(6):1733-47. doi: 10.3892/ijo.2012.1408 [Crossref] [ Google Scholar]
- Viviani S, Jack A, Bah E, Montesano R. [Hepatocellular carcinoma: a preventable cancer]. Epidemiol Prev 1997;21(2):129-36. [Italian].
- Kryczka W, Chrapek M, Paluch K, Zarebska-Michaluk D, Urbaniak A. [Rate of liver fibrosis progression among patients with chronic hepatitis C in Poland]. Pol Arch Med Wewn 2003;110(2):869-75. [Polish].
- Sakhuja P, Malhotra V, Gondal R, Sarin SK, Thakur V. Histological profile of liver disease in patients with dual hepatitis B and C virus infection. Indian J Pathol Microbiol 2003; 46(4):555-8. [ Google Scholar]
- Lee JY, Kim JY, Lee YG, Byeon SE, Kim BH, Rhee MH. In vitro immunoregulatory effects of Korean mistletoe lectin on functional activation of monocytic and macrophage-like cells. Biol Pharm Bull 2007; 30(11):2043-51. doi: 10.1248/bpb.30.2043 [Crossref] [ Google Scholar]
- Kim SJ, Um JY, Lee JY. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-κB activation in mouse peritoneal macrophages. Am J Chin Med 2011; 39(1):171-181. doi: 10.1142/S0192415X11008737 [Crossref] [ Google Scholar]
- Lopetuso LR, Scaldaferri F, Pizarro TT. Emerging role of the interleukin (IL)-33/ST2 axis in gut mucosal wound healing and fibrosis. Fibrogenesis Tissue Repair 2012; 5(1):18. doi: 10.1186/1755-1536-5-18 [Crossref] [ Google Scholar]
- Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol 2011; 22(11):2057-67. doi: 10.1681/asn.2010091011 [Crossref] [ Google Scholar]
- Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 2011; 10:150. doi: 10.1186/1476-4598-10-150 [Crossref] [ Google Scholar]
- Knab AM, Nieman DC, Gillitt ND, Shanely RA, Cialdella-Kam L, Henson DA. Effects of a flavonoid-rich juice on inflammation, oxidative stress, and immunity in elite swimmers: a metabolomics-based approach. Int J Sport Nutr Exerc Metab 2013; 23(2):150-60. doi: 10.1123/ijsnem.23.2.150 [Crossref] [ Google Scholar]
- Selvam C, Jachak SM, Bhutani KK. Cyclooxygenase inhibitory flavonoids from the stem bark of Semecarpus anacardium Linn. Phytother Res 2004; 18(7):582-4. doi: 10.1002/ptr.1492 [Crossref] [ Google Scholar]