Biomed Res Bull. 1(3):113-117.
doi: 10.34172/biomedrb.2023.22
Review Article
Generation and Immuno-inflammatory Checkpoints in Oral Cavity Cancers
Leilasadat Hatamnezhad 1, * 
Author information:
1Department of Dermatology, Sina Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Oral malignancies are responsible for a considerable portion of cancer-related deaths worldwide. Even though survival rates have increased recently, new treatments are being explored to slow the advancement of the disease and enhance outcomes, especially in cases of oral cavity squamous cell carcinoma (OSCC) and oral potentially malignant diseases (OPMDs). Immunotherapy is a novel therapeutic approach that targets immune checkpoint molecules such as programmed cell death protein-1 (PD-1) and its ligand programmed death-ligand 1 (PD-L1), cytotoxic T-lymphocyte antigen 4, lymphocyte-activated gene 3, and T cell immunoglobulin mucin 3 in order to enhance the host’s immune response against malignancies and impede the growth and metastasis of cancer cells. Accordingly, a systematic review was performed by scanning five databases for keywords related to immune checkpoint inhibitors, along with oral malignancies, oral pathologies, and OPMDs, in order to describe the current state of their use and efficacy in these disorders. For this purpose, 644 unique publications published between 2004 and 2019 were found, 76 of which were judged to be appropriate for the study and produced 8826 samples. PD-1 and PD-L1 are expressed in most OPMD and OSCC samples, and their expression is associated with worse survival rates and greater rates of progression. Two immunotherapy drugs targeting PD-1, namely, pembrolizumab and nivolumab, have been demonstrated to enhance disease outcomes and increase survival rates, especially when combined with radiation or chemotherapy. Despite the equivocal nature of the available data, there is support for the prognostic and predictive usefulness of immune checkpoint molecules, notably PD-L1, and multiple studies support the useful use of immune checkpoint inhibitors in the management of OSCC.
Keywords: Generation, Oral cancer, Immune-check point
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The oral cavity extends from the vermilion border of the lips to the superior junction of the hard and soft palates, as well as the inferior circumvallate papillae of the tongue.1 The lip, oral tongue, floor of the mouth, buccal mucosa, upper and lower gums, retromolar trigone, and hard palate are the anatomical subsites that together comprise the oral cavity. Despite their proximity, these subsites have unique anatomical features that should be taken into account when designing oncologic therapy.2 The fifth decade of life is when oral cancer usually manifests itself in most men. About 1.5% of individuals will experience another synchronous primary in the lung, esophagus, or oral cavity of the aero-digestive tract. Nearly 10%–40% of metachronous cancers arise in the first ten years after the treatment of the index primary.3,4 Regular post-therapy surveillance and lifestyle adjustments are therefore crucial secondary preventive measures. Squamous cell carcinomas account for more than 90% of all oral cancer cases. Other malignant tumors can also arise from the epithelium, connective tissue, small salivary glands, lymphoid tissue, melanocytes, and metastasis of a distant tumor. Numerous premalignant lesions have been connected to squamous cell carcinoma (SCC). Among the most common premalignant lesions, leukoplakia, erythroplakia, oral lichen planus, and oral submucous fibrosis all have different degrees of risk for developing into malignant transformation 5. Based on the degree of dysplasia, premalignant lesions are classified as carcinoma in situ, mild, moderate, severe, and severe, according to the World Health Organization.
T Cell Activation and Regulation
Signals that are produced when the T cell receptor identifies the major histocompatibility complex (MHC) antigen are responsible for T cell activation. As stimulatory factors, the B molecule on the surface of the antigen-presenting cell (APC) and the CD28 molecule on the surface of the T cell carry out these signals. Checkpoints both centrally and peripherally control T lymphocytes. Bidirectional T cell activation generates an inhibitory pathway that ultimately reduces the potency of the T cell response.6
Cytotoxic T Lymphocyte Antigen 4
CTLA-4, or cytotoxic T lymphocyte antigen 4, which is mostly expressed in T cells, was identified as the first immune checkpoint receptor to be targeted. Activated B cells, monocytes, granulocytes, and dendritic cells (DCs) express these factors at lower levels. Inducing T cell malfunction and contributing to the negative regulation of the immune response, CTLA-4 can bind the B7 protein. CTLA-4 has an inhibitory effect on normal tissues without causing undue harm, and cancer cells release transforming growth factor-beta (TGF-β), which can stimulate CTLA-4 expression and cause T cell exhaustion, which impairs T cell activity and suppresses the immune system. T cell function is lost as a result of CD28’s greater affinity for CTLA-4 on T cell surfaces than for CD80 or CD86.7-9
Two ligands expressed on the surface of APC, CD80 and CD86, are bound by both CTLA-4 and CD28. Furthermore, CTLA-4 expression rises in response to T cell activation to the point where it finally blocks co-stimulation and deactivates T cells. Tregs express CTLA-4 as well. Some of the characteristics of CTLA-4 make it occasionally localize to the plasma membrane. About 90% of CTLA-4 is found inside cells. CTLA-4 induces endocytosis as well. Research indicates that lysosomal blockage has the ability to prevent CTLA-4 degradation10 (Figure 1).
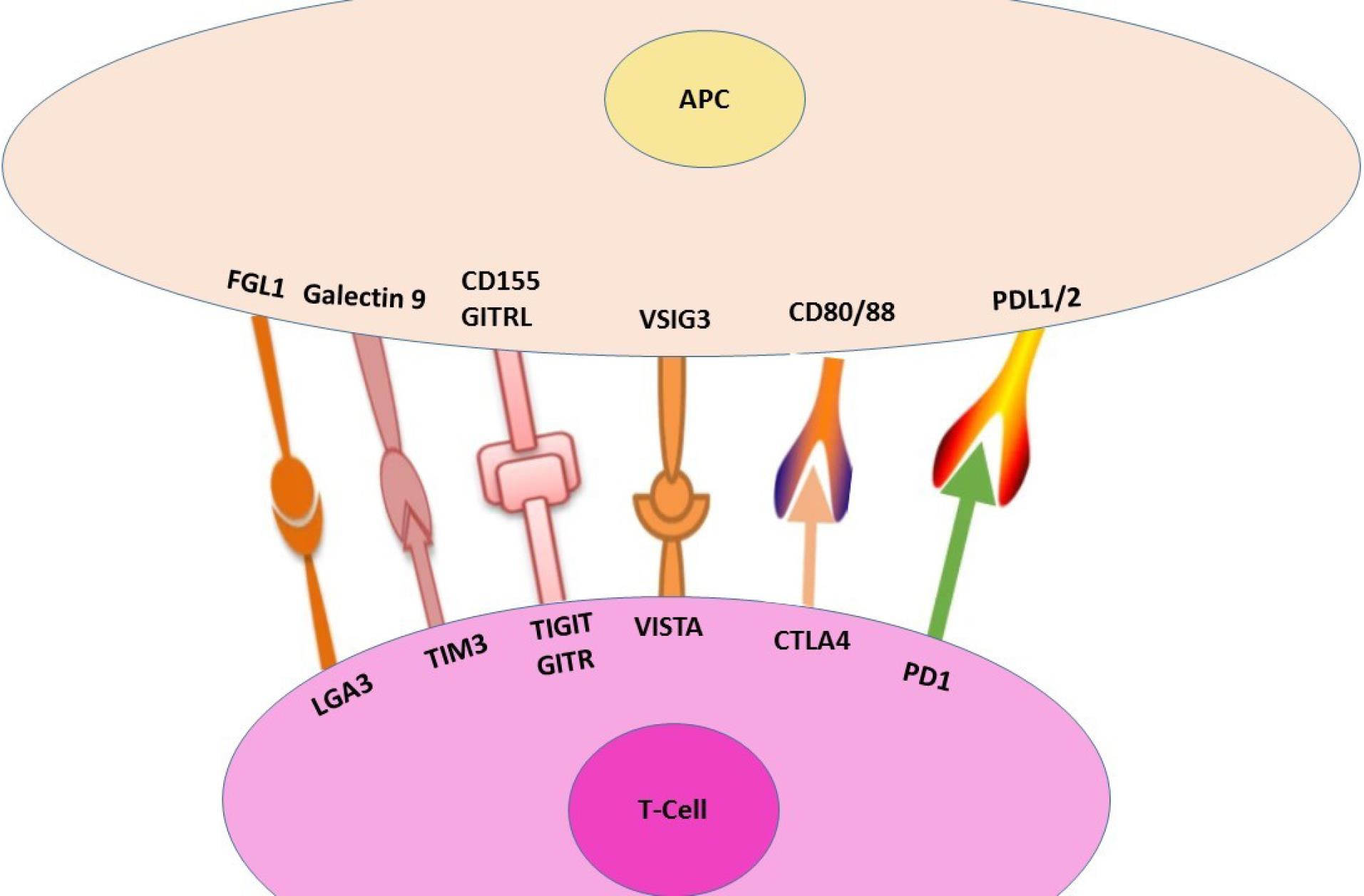
Figure 1.
A Summary of Immune-check Points
.
A Summary of Immune-check Points
Programmed Death Ligand 1/Programmed Cell Death Protein-1
Activated T cells and B cells express PD-1, a member of the CD28 receptor family. Monocytes and a tiny portion of thymocytes also contain this protein. Activated lymphocytes, endothelium, and epithelial APCs express PD-L1 and PD-L2, two ligands for PD-1, although PD-L1 is highly induced in hematological and solid cancers. The tumor cell overexpression of PD-L1 can encourage the growth of new tumors. Patients with poor prognoses and high tumor grades are closely correlated with high expression of PD-L1 in tumor cells. PD-1/PD-L1 signaling is triggered during inflammatory responses. Although Tregs’ capacity to mediate immunological tolerance is hindered when PD-1 is inhibited, this does not imply that PD-1 can directly control Treg function. In addition to diminishing antitumor T cell function, PD-L1 modifies the relationship between DCs, myeloid-derived suppressor cells, and Tregs. PD-1 and CTLA-4 share structural similarities and are members of the same protein family. DCs and activated macrophages both express the PD-L2 molecule. Cytokine production is suppressed, and lymphocyte proliferation is reduced by binding PD-L1 to PD-1. T cells become phosphorylated when PD1 binds to PDL-1, which in turn induces downstream kinase proteins to become dephosphorylated.11,12
T Cell Immunoglobulin Mucin 3
Th2 cells do not express TIM-3; only cluster of differentiation 4 (CD4) + T helper 1 (Th1) lymphocytes express it. Tregs, DCs, monocytes, mast cells, natural killer (NK) cells, and tumor-infiltrating lymphocytes express it as well. Additionally, tumor cells, including melanoma and B-cell lymphoma cells, have TIM-3 on them. Research has indicated that T cell tolerance can be regulated by TIM-3 and its ligands. Galectin-9, a member of the galectin family, is the primary ligand for TIM-3. It has the ability to control a variety of biological processes in tumor cells, including adhesion, apoptosis, and aggregation. Moreover, it can impair Th1 and Th17 cell function while ultimately speeding up the Th1 cell apoptotic process. By secreting interferon‐gamma (IFN-γ), TIM-3 + CD4 + cells exert anti-tumor actions in the early phases of tumor growth. Though research indicates that TIM-3 expression is not correlated with metastasis—which is not the case in lymph nodes—it is elevated in carcinomas and adenocarcinomas. Excessive production of TIM-3 leads to effector T cell fatigue, which can impede the antitumor immune response and tumor clearance. Tumorigenesis is dramatically reduced, and antitumor immune responses are enhanced when CD4 + and CD8 + T cell function is increased and suppressor cell activity is decreased.13-15
Lymphocyte-activated Gene 3
The immunoglobulin (Ig) superfamily, which includes this protein, is mostly expressed on activated T cells, while it can also be expressed on B cells, NK cells, and plasmacytoid DCs. LAG-3 acts similarly to CTLA-4 and PD-1.37, negatively modulating T cell proliferation, activation, and homeostasis. LAG-3 is essential to Tregs’ ability to serve as inhibitors. LAG-3 has a higher affinity for MHC-II and shares the same protein sequence as the CD4 receptor. The connection between LAG-3 and MHC-II is not necessary for its effect on CD8 + T cell activity. Another ligand for LAG-3 is thought to be liver and lymph node sinusoidal endothelial cell C-type lectin (LSECtin), which is a member of the DC signaling family. LSECtin inhibits the anti-tumor immune response by binding to LAG-3 and reducing IFN-γ production in cancer. LAG-3 is essential for maintaining T cell homeostasis and is mostly expressed in Tregs. According to research, fibrinogen-like protein 1 (FGL1), a new ligand for LAG-3, functions as MHC-II independently as the primary functional ligand for LAG-3. FGL1 enhances T cell activity, inhibits antigen-specific T cell activation, and fortifies anti-tumor immunity.16-18
T Cell Immunoglobulin and Receptor Tyrosine-based Inhibitory Motif
TIGIT belongs to the family of poliovirus receptor/nectin, which is made up of four domains, including the type 1 transmembrane domain, the extracellular Ig (IgV) variable region domain, the classical ITIM, and the Ig tyrosine d motif. TIGI lymphocytes express this immunoglobulin, particularly NK cells, follicular helper CD4 + T cells, effector CD8 + T cells, and regulatory and effector CD4 + T cells. Moreover, endothelial cells, fibroblasts, DCs, and tumor cells all have high expression levels of this cell surface receptor.19,20
Glucocorticoid-Induced Tumor Necrosis Factor Receptor Family Gene
Effector T cells, NK cells, and CD25 + CD4 + Tregs all express GITR, a new member of the TNFR superfamily. Treg recruitment is inhibited when GITR binds to its ligand. It reduces their inhibitory capacity and increases Nuclear factor kappa B (NF-κB) signaling and the mitogen-activated protein kinase/signal-regulated kinase pathway. This gene triggers the release of pro-inflammatory cytokines, boosts T cell proliferation, and improves anti-tumor activity.21-24
Immunoglobulin Domain V Suppressor of T Cell Activation
This molecule is known as Gi24, or embryonic stem cell differentiation 1, and functions similarly to PD-L1 in terms of function and potency in suppressing T cell activation. Numerous tumor models show that VISTA enhances antitumor immune blockade and is strongly expressed on tumor-infiltrating leukocytes.25
V Suppressor of T Cell Activation and Programmed Cell Death Protein-1 Inhibit T Cells and Modulate T Cell Responses
On activated T cells, the interaction between ligand and VISTA suppresses T cell growth and chemokine and cytokine production. Blocking the VSIG3 pathway and its ligand may be a novel approach to cancer immunotherapy, as evidenced by the inhibition of ligands on activated T cells and the high expression of ligand in colorectal adenocarcinoma, hepatocellular carcinoma, and intestinal-type gastric cancer.26-28
Anti-inflammatory Function
Research has demonstrated that inflammation, which results in tissue damage and the release of particular inflammatory cytokines, raises the aggressiveness of oral malignancies. According to previous research, oral cavity squamous cell carcinoma (OSCC) can prevent cancer from becoming malignant by expressing pro- or anti-inflammatory cytokines (TGF-β1, interleukin [IL-10], IL-4, or IFN-γ, monocyte chemoattractant protein 1). Th cells, or Th cells, are functionally categorized as Th1, Th2, and Th17 cells and are mostly involved in tumor immunology.29 While most Th1 cytokines, including IFN-γ, are classified as protumors, Th2 cytokines (IL-4, IL-5, and IL-10) are considered anti-inflammatory and are frequently linked to protumor activity. Patients with OSCC had significantly greater serum levels of IL-17A, TGF-β1, IL-4, and IL-10 than the control group, but comparatively lower levels of IL-2 and IFN-γ. In patients with OSCC, the negative regulation of NK cells is linked to the increased production of IL-10 and TGF-β1, as well as reduced IFN-γ.30,31
Anti-inflammatory and Anti-tumor Cytokines
TGF-β and IL-10 are anti-inflammatory and immunosuppressive cytokines that lead to immunological suppression of neoplastic cells. They are also anti-tumor cytokines. The overexpression of IL-10 and TGF-β2 is, in fact, linked to a worse prognosis for OSCCs. It suppresses dendritic and macrophage cells, controls regulatory T cell development, and builds tumor cells’ resistance to the effects of cytotoxic T lymphocytes. IL-4, an additional anti-inflammatory cytokine, is regarded as proinflammatory as well.32,33 IL-17 is an inflammatory cytokine that is mostly released by neutrophils and type 17 T-helper cells. In OSCC, there has also been evidence of increased IL-17 expression. The advancement of tongue cancer is linked to IL-17 overexpression. The expression of IL-17 protein in the OSCC tissue is linked to a worse prognosis, including metastasis, recurrence, clinical stages, and T classification.33
Therapeutic Approaches
A humanized IL-1α neutralizing antibody called MABp1 (XBiotech Inc.) has been shown to enhance clinical outcomes and survival rates in patients with advanced non-small cell lung cancer, ovarian cancer, and other resistant malignancies.34-36
Targeting Tumor-Associated Macrophages
A novel treatment method is provided by manipulating protumorogenic M2 polarization to the anticancer M1 macrophage phenotype. Using poly (I:C) to stimulate TLR3/Toll-IL-1 receptor domain-containing adapter molecule 1, many proinflammatory cytokines, including IL-1β, are secreted more quickly, and M1 macrophage polarization is accelerated.37 Tumor-conditioned macrophages can be effectively stimulated to produce cytotoxic activity against cancer cells by the TLR3 agonist poly (I:C).38
Conclusion
The tumor microenvironment (TME) of OSCC contains a variety of immune modulators, such as cytokines, immune checkpoint inhibitors, and cellular elements. Within the TME, extrinsic and internal processes regulate these substances and cellular constituents. Targeting these processes with particular antibodies, miRNAs, and extracellular vesicles produced from T cells are some of the treatments available. Bisphosphonates can be used to stimulate alternate TAM differentiation as a novel treatment strategy. However, when it comes to optimizing treatment strategies, the elements of pre-tumor immunity remain incompletely known. Immunology and anesthesia transplantation may provide novel therapeutic benefits.
Competing Interests
None.
Ethical Approval
Not applicable.
Funding
None.
References
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28(19):3167-75. doi: 10.1200/jco.2009.26.7609 [Crossref] [ Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366(26):2455-65. doi: 10.1056/NEJMoa1200694 [Crossref] [ Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369(2):134-44. doi: 10.1056/NEJMoa1305133 [Crossref] [ Google Scholar]
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32(10):1020-30. doi: 10.1200/jco.2013.53.0105 [Crossref] [ Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369(2):122-33. doi: 10.1056/NEJMoa1302369 [Crossref] [ Google Scholar]
- National Cancer Institute. Cancer Stat Facts: Melanoma of the Skin. Available from: http://seer.cancer.gov/statfacts/html/melan.html. Accessed March 15, 2015.
- Medical Research Council Renal Cancer Collaborators. Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Lancet 1999; 353(9146):14-7. [ Google Scholar]
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994; 271(12):907-13. [ Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711-23. doi: 10.1056/NEJMoa1003466 [Crossref] [ Google Scholar]
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33(17):1889-94. doi: 10.1200/jco.2014.56.2736 [Crossref] [ Google Scholar]
- Joseph RW, Eckel-Passow JE, Sharma R, Liu P, Parker A, Jakob J. Characterizing the clinical benefit of ipilimumab in patients who progressed on high-dose IL-2. J Immunother 2012; 35(9):711-5. doi: 10.1097/CJI.0b013e3182742c27 [Crossref] [ Google Scholar]
- Payne R, Glenn L, Hoen H, Richards B, Smith JW, 2nd 2nd, Lufkin R. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a Community Hospital Biotherapy Program. J Immunother Cancer 2014; 2:13. doi: 10.1186/2051-1426-2-13 [Crossref] [ Google Scholar]
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15(23):7412-20. doi: 10.1158/1078-0432.ccr-09-1624 [Crossref] [ Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372(4):320-30. doi: 10.1056/NEJMoa1412082 [Crossref] [ Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11(11):3887-95. doi: 10.1002/j.1460-2075.1992.tb05481.x [Crossref] [ Google Scholar]
- Freeman GJ. Structures of PD-1 with its ligands: sideways and dancing cheek to cheek. Proc Natl Acad Sci U S A 2008; 105(30):10275-6. doi: 10.1073/pnas.0805459105 [Crossref] [ Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192(7):1027-34. doi: 10.1084/jem.192.7.1027 [Crossref] [ Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001; 2(3):261-8. doi: 10.1038/85330 [Crossref] [ Google Scholar]
- Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8 + T cell activation and cytolysis. Eur J Immunol 2003; 33(11):3117-26. doi: 10.1002/eji.200324270 [Crossref] [ Google Scholar]
- Zinselmeyer BH, Heydari S, Sacristán C, Nayak D, Cammer M, Herz J. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med 2013; 210(4):757-74. doi: 10.1084/jem.20121416 [Crossref] [ Google Scholar]
- Honda T, Egen JG, Lämmermann T, Kastenmüller W, Torabi-Parizi P, Germain RN. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity 2014; 40(2):235-47. doi: 10.1016/j.immuni.2013.11.017 [Crossref] [ Google Scholar]
- Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger LR. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp Cell Res 1997; 232(1):25-8. doi: 10.1006/excr.1997.3493 [Crossref] [ Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998; 188(12):2205-13. doi: 10.1084/jem.188.12.2205 [Crossref] [ Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23:515-48. doi: 10.1146/annurev.immunol.23.021704.115611 [Crossref] [ Google Scholar]
- Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol 1999; 162(10):5784-91. [ Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704. doi: 10.1146/annurev.immunol.26.021607.090331 [Crossref] [ Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005; 25(21):9543-53. doi: 10.1128/mcb.25.21.9543-9553.2005 [Crossref] [ Google Scholar]
- Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A 2005; 102(33):11823-8. doi: 10.1073/pnas.0505497102 [Crossref] [ Google Scholar]
- Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 2003; 9(12):1477-83. doi: 10.1038/nm955 [Crossref] [ Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11(2):141-51. doi: 10.1016/s1074-7613(00)80089-8 [Crossref] [ Google Scholar]
- Kasagi S, Kawano S, Okazaki T, Honjo T, Morinobu A, Hatachi S. Anti-programmed cell death 1 antibody reduces CD4 + PD-1 + T cells and relieves the lupus-like nephritis of NZB/W F1 mice. J Immunol 2010; 184(5):2337-47. doi: 10.4049/jimmunol.0901652 [Crossref] [ Google Scholar]
- Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med 1999; 190(5):733-40. doi: 10.1084/jem.190.5.733 [Crossref] [ Google Scholar]
- Nielsen C, Ohm-Laursen L, Barington T, Husby S, Lillevang ST. Alternative splice variants of the human PD-1 gene. Cell Immunol 2005; 235(2):109-16. doi: 10.1016/j.cellimm.2005.07.007 [Crossref] [ Google Scholar]
- Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003; 423(6939):506-11. doi: 10.1038/nature01621 [Crossref] [ Google Scholar]
- Wan B, Nie H, Liu A, Feng G, He D, Xu R. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol 2006; 177(12):8844-50. doi: 10.4049/jimmunol.177.12.8844 [Crossref] [ Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443(7109):350-4. doi: 10.1038/nature05115 [Crossref] [ Google Scholar]
- Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med 2003; 198(1):39-50. doi: 10.1084/jem.20022235 [Crossref] [ Google Scholar]
- Jun H, Seo SK, Jeong HY, Seo HM, Zhu G, Chen L. B7-H1 (CD274) inhibits the development of herpetic stromal keratitis (HSK). FEBS Lett 2005; 579(27):6259-64. doi: 10.1016/j.febslet.2005.09.098 [Crossref] [ Google Scholar]