Biomed Res Bull. 1(1):3-6.
doi: 10.34172/biomedrb.2023.02
Original Article
The Levels of mRNA for Pro- and Anti-inflammatory Cytokines and B Cell Amounts in Patients With Lupus Erythematosus
Elham Jarollah Fattahi 1  , Vadud Nouruzi 1, Leila Mahboobi 1, 2, *
, Vadud Nouruzi 1, Leila Mahboobi 1, 2, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Pediatric, Faculty of Medicine, Ardabil University of Medical Siences,Ardabil, Iran
Abstract
Background:
Systemic lupus erythematosus, is the most prevalent form of lupus. SLE is an immune system sickness wherein the resistant framework goes after its own tissues, causing boundless aggravation and tissue harm in the impacted organs. The purpose of this study was to examine the levels of mRNA for pro- and anti-inflammatory cytokines and B cell amounts in patients with lupus erythematosus.
Methods:
This case-control study was performed on 6 patients of those with lupus who were higher than 18 years old and referred to Sina Hospital Clinic to be visited and examined during 2021-2022, and their lupus illness was definitely diagnosed by rheumatology and a dermatologist. The control and patient groups were analyzed using a paired t test.
Results:
The results of the present study demonstrated that the number of B cells in the blood samples of the patients was lower than that of the control group. Based on the results, the levels of interleukin (IL)-17, IL-6, and IL-1 in the patient group were higher than in the control group (P<0.005), indicating an increase in inflammation in these patients. Finally, the amount of inhibitory cytokine IL-10 was decreased in these patients.
Conclusion:
Overall, pro-inflammatory cytokines were more secreted in patients with lupus and inhibitory cytokines were less; thus, cytokine can be used as a biomarker of inflammation in these patients.
Keywords: B- cell lymphocytes, mRNA, Lupus
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Lupus is a systemic and chronic autoimmune disease with significant heterogeneity in clinical symptoms, and the main cause of this disease remains unknown. However, the evidence indicates that lupus is a consequence of the intervention of environmental, immunological, and genetic factors, finally, causing a breakdown of tolerance toward self-antigens and the start of an abnormal immune response to them and tissue damage.1-4 It seems that the basic defect in systematic lupus erythematosus (SLE) is the dysfunction of T lymphocytes in B lymphocyte control, leading to the activation of polyclonal B lymphocytes and the production of a large number of autoantibodies.5 This autoantibody can damage various tissues directly or as a result of immune complex deposition.6-8 The increase in the expression of inflammatory cytokines increases the proliferation of autoreactive B cells and the production of more autoantibodies. The overexpression of pro-inflammatory cytokines plays a role in the pathogenesis of lupus.9-12 The excessive increase of interleukin (IL)-17 cytokine is also associated with an increase in the Th17 subset of T cells. IL-6 exerts an important role in B cell hyperactivity and immunopathology of SLE and may directly contribute to mediating tissue damage. Based on these data, it was proposed that the inhibition of IL-6 in humans can improve systemic and local systemic inflammation in lupus.12-14 Accordingly, this study aimed to evaluate inflammatory and anti-inflammatory cytokines in lupus patients.
Materials and Methods
Patient and Cell Isolation
Overall, 24 samples were selected from lupus patients (n = 12) and healthy people (n = 12) as available sampling. Then, 5 cc of venous blood was taken from each patient. Monocyte cells were isolated and cultured by the peripheral blood mononuclear cell method. The mRNA was extracted with a TRIzol reagent based on the protocol. Patients suffering from lupus disease without treatment were chosen as the inclusion criteria. On the other hand, patients having a chronic disease or treated lupus were excluded from the study.
Real-time Polymerase Chain Reaction
The cDNA of the cells of the patients and the control group was diluted in a ratio of 1-6 using distilled water. Primers were prepared to separately determine the expression levels of IL-17, IL10, IL-6, and IL-1 genes. The tube containing the material was centrifuged for a few seconds to thoroughly mix and homogenize the material. The cDNA was added to the wells, and the set time and temperature schedule for the RT-PCR included 10 minutes at 95°C (time and temperature of enzyme activation and the initial formation), 15 seconds at 95°C (time and temperature of each cycle), 60 seconds at 62°C (time and temperature of extension and annealing of each cycle), and reading of the irradiated fluorescent at the end of each cycle. The number of cycles was also 40 times. To increase accuracy, each reaction was performed in duplicate (for repetition). The primers are provided in Table 1.
Table 1.
The Primers of Real-Time Polymerase Chain Reaction
|
Primer
|
|
Sequence
|
| IL-1 primer |
F |
3' AACCGGGTTTAGGGCTATG5' |
| R |
3' GAAAGGGCCCCTTAACAATC5' |
| IL-17 primer |
F |
3' CCTTAGGTTGGGCTCTATG5' |
| R |
3' GAAAAGCCCCTTTATGATC5' |
| IL-6 primer |
F |
3' CCCTAGGGTTTAGGGCTATG5' |
| R |
3' AAGGTAGCCCCTTAACAATC5' |
| IL-10 primer |
F |
3' AACCGGTTCAATGGCTATG5' |
| R |
3' GAAAGCTAGGCCCTTAAATC5' |
| B-actin primer |
F |
3' ATTACCGGGTTGGGCTATG5' |
| R |
3' AATAGGGCCGTCAACAATC5' |
Flow Cytometry
The isolated monocytes were washed with phosphate-buffered saline and added to flow cytometry tubes. The samples were divided into two parts called the test and isotype tubes. In addition, CD19 primary antibody was diluted and the IgGT-PE isotype was added to the isotype tube at a ratio of 1:100. Further, CD4 primary antibody was diluted and the IgGT-APC isotype was added to the isotype tube with a ratio of 1:50, and CD3 primary antibody diluted with a ratio of 1:100 was added. Incubation was performed at 4°C for 1 hour in the dark. Centrifugation was performed for 5 minutes at 1500 rpm, and diluted secondary antibody 488-IHouse anti-human Alexa was added to both test and isotype tubes at a ratio of 1:500. Incubation was conducted at 4°C for 1 hour in the dark, and after washing and centrifugation, it was read by flow cytometry and analyzed with FlowJo software, version 7.6.1.
Data Analysis
The obtained data were statistically analyzed by Excel and SPSS (version 16) software programs. P > 0.05 was considered significant. Quantitative information is presented as means ± standard deviations (SD). One-sample Kolmogorov-Smirnov test was used to check the normal distribution of quantitative data.
Results
Expression of Pro-inflammatory Cytokines IL-1, IL-17, IL-6, and IL-10 in the Monocytes of Lupus Patients Compared to the Control Group
The analysis of the level of IL-1 expression in 12 lupus monocyte cells and 12 normal monocyte cells represented a significant increase in IL-1 expression in patient samples compared to control samples (P = 0.001). The evaluation of the amount of IL-17 in 12 lupus samples and 12 normal samples with a t-test demonstrated a significant increase in IL-17 in patient samples in comparison to normal samples (P < 0.001). Examining the intensity of IL-6 expression revealed a significant increase in IL-6 expression in patient samples compared to the control samples (P < 0.001, Figures 1A, 1B, and 1C).
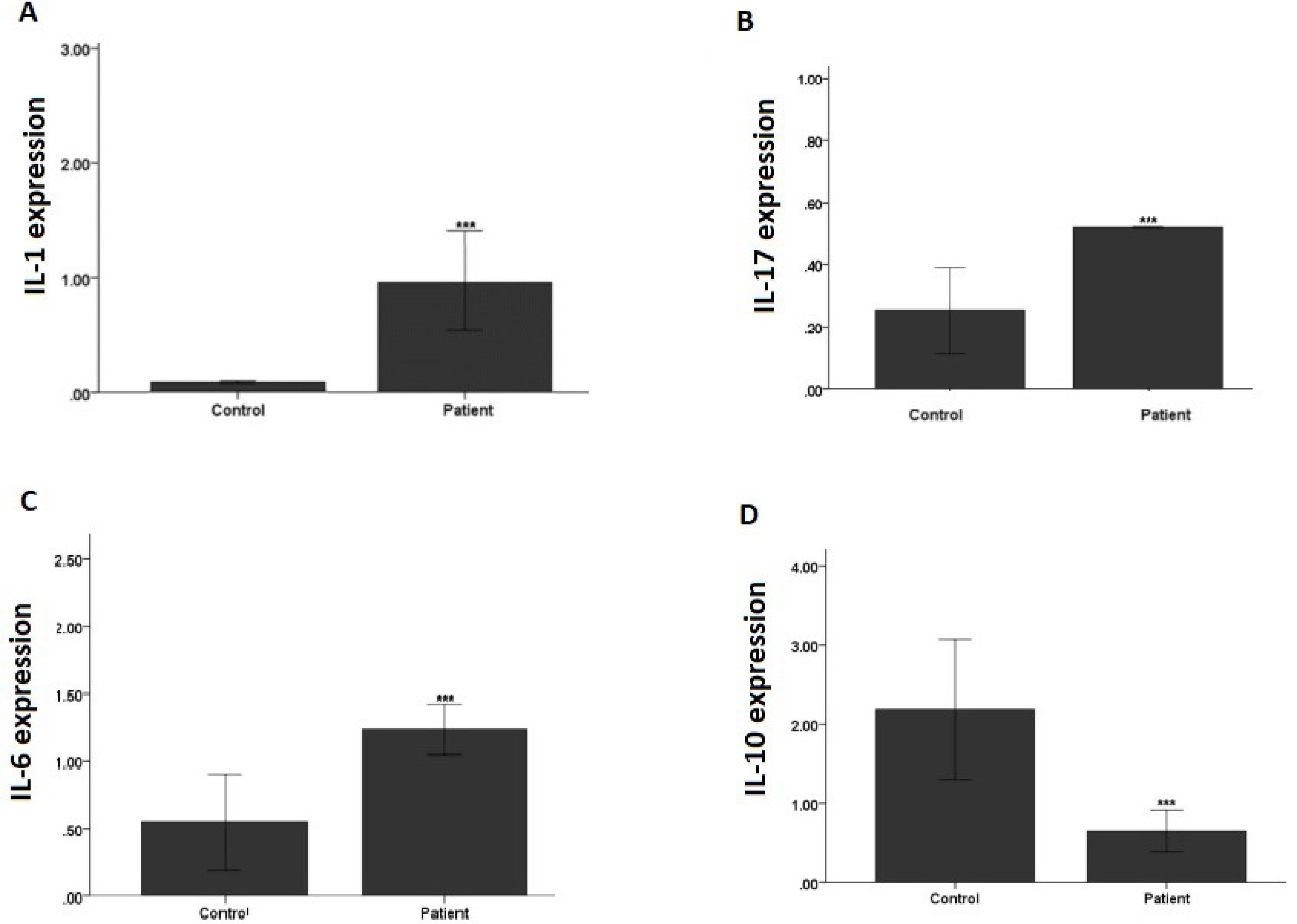
Figure 1.
Cytokines Expression Levels in Systematic Lupus Erythematosus, (A) IL-1, (B) IL-17, (C) IL-6, and (D) IL-10. Note. IL: interleukin
.
Cytokines Expression Levels in Systematic Lupus Erythematosus, (A) IL-1, (B) IL-17, (C) IL-6, and (D) IL-10. Note. IL: interleukin
Based on the evaluation of the level of IL-10 expression, a significant decrease was found in IL-10 expression in patient samples compared to the control (Figure 1D).
The Results of B Cell Expression
According to data in Table 2, the number of B cells in the patient and normal blood samples was measured with flow cytometry and analyzed by a t-test, and the test results showed a significant increase in the expression of CD19 in lupus samples (Table 2).
Table 2.
The B Cell Expression
|
|
Normal
|
Patient
|
| Mean ± SD |
2.4 ± 10.08 |
2.01 ± 26.8 |
|
P value |
< 0.005 |
< 0.005 |
Note. SD: Standard deviation.
Discussion
The role of proinflammatory cytokines in the pathogenesis of SLE is still controversial. Several studies investigated the cytokine profile of SLE patients in vivo and in vitro.15-17 However, the findings of studies in this area are contradictory. Cytokines are essential molecules involved in the differentiation, maturation, and activation of cells and thus have a naturally significant impact on the immune inflammatory response. In autoimmune diseases, cytokines may not only play a role in immune dysregulation but also in local inflammatory processes, ultimately leading to tissue destruction. Similar to the results of Brugos et al,18 Umar et al,19 and Cigni et al,20 while contrary to present study results, the level of IL-1 in the SLE group was significantly higher than the control group in the current study. According to this finding, IL-1 can be used as an inflammatory marker of SLE disease activity. The results of our study are in line with those of Wong et al, Ouyang et al, Yang et al, Tang et al, and Zhao et al, demonstrating that the level of IL-17 in the patient group was higher than that of the control group, confirming an increase in inflammation in these patients.21-26 However, other studies suggested that IL-6 serum levels may not be associated with SLE disease.27-30 Similar to the study by Uchida et al,31 and Yao et al,32 while contrary to the study by Ishida et al33 and Abd Elazeem et al,34 Wardowska et al,35 and Liu et al,36 the serum levels of IL-10 were significantly decreased in patients with SLE. Our results conform to the findings of Korganow et al and Wardowska et al, representing that the expression of CD19, which was considered a B-cell diagnosis marker in the present study, was higher than that of the normal groups.35,37
Conclusion
Our findings revealed that in terms of inflammation, pro-inflammatory cytokines IL-6, IL-17, and IL-1 had a significant increase in the patient group compared to the control group, but the level of IL-10 in the patient group was lower than the control group.
Acknowledgments
The authors would like to thank the Immunology Research Center of Tabriz University of Medical Sciences for cooperating in conducting the study.
Authors’ Contribution
Conceptualization: Elham Jarollah Fattahi, Vadud Nouruzi, Leila Mahboobi.
Data Curation: Elham Jarollah Fattahi, Vadud Nouruzi, Leila Mahboobi.
Formal Analysis: Elham Jarollah Fattahi, Vadud Nouruzi, Leila Mahboobi.
Funding Acquisition: Self Fund.
Methodology: Elham Jarollah Fattahi, Vadud Nouruzi, Leila Mahboobi.
Project Administration: Leila Mahboobi.
Supervision: Leila Mahboobi.
Writing — Original Draft: Elham Jarollah Fattahi.
Competing Interests
None.
Ethical Approval
The study was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1401.633).
Funding
Self-funded.
References
- Dörner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet 2019; 393(10188):2344-58. doi: 10.1016/s0140-6736(19)30546-x [Crossref] [ Google Scholar]
- Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers 2020; 6(1):7. doi: 10.1038/s41572-019-0141-9 [Crossref] [ Google Scholar]
- Barbhaiya M, Costenbader KH. Ultraviolet radiation and systemic lupus erythematosus. Lupus 2014; 23(6):588-95. doi: 10.1177/0961203314530488 [Crossref] [ Google Scholar]
- Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med 2012; 18(6):871-82. doi: 10.1038/nm.2752 [Crossref] [ Google Scholar]
- Gergianaki I, Bortoluzzi A, Bertsias G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2018; 32(2):188-205. doi: 10.1016/j.berh.2018.09.004 [Crossref] [ Google Scholar]
- Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol 2016; 12(10):605-20. doi: 10.1038/nrrheum.2016.137 [Crossref] [ Google Scholar]
- Bertsias G, Karampli E, Sidiropoulos P, Gergianaki I, Drosos A, Sakkas L. Clinical and financial burden of active lupus in Greece: a nationwide study. Lupus 2016; 25(12):1385-94. doi: 10.1177/0961203316642310 [Crossref] [ Google Scholar]
- Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis 2021; 80(1):14-25. doi: 10.1136/annrheumdis-2020-218272 [Crossref] [ Google Scholar]
- Bertsias GK, Salmon JE, Boumpas DT. Therapeutic opportunities in systemic lupus erythematosus: state of the art and prospects for the new decade. Ann Rheum Dis 2010; 69(9):1603-11. doi: 10.1136/ard.2010.135186 [Crossref] [ Google Scholar]
- Arnaud L, Mertz P, Gavand PE, Martin T, Chasset F, Tebacher-Alt M. Drug-induced systemic lupus: revisiting the ever-changing spectrum of the disease using the WHO pharmacovigilance database. Ann Rheum Dis 2019; 78(4):504-8. doi: 10.1136/annrheumdis-2018-214598 [Crossref] [ Google Scholar]
- Barbhaiya M, Tedeschi SK, Lu B, Malspeis S, Kreps D, Sparks JA. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses’ Health Study cohorts. Ann Rheum Dis 2018; 77(2):196-202. doi: 10.1136/annrheumdis-2017-211675 [Crossref] [ Google Scholar]
- Gustafsson JT, Gunnarsson I, Källberg H, Pettersson S, Zickert A, Vikerfors A. Cigarette smoking, antiphospholipid antibodies and vascular events in systemic lupus erythematosus. Ann Rheum Dis 2015; 74(8):1537-43. doi: 10.1136/annrheumdis-2013-205159 [Crossref] [ Google Scholar]
- Bertsias GK, Boumpas DT. Pathogenesis, diagnosis and management of neuropsychiatric SLE manifestations. Nat Rev Rheumatol 2010; 6(6):358-367. doi: 10.1038/nrrheum.2010.62 [Crossref] [ Google Scholar]
- Smith PP, Gordon C. Systemic lupus erythematosus: clinical presentations. Autoimmun Rev 2010; 10(1):43-5. doi: 10.1016/j.autrev.2010.08.016 [Crossref] [ Google Scholar]
- Shamsoddini S, Fekri A, Ebrahimi H, Zeinodini M. Survival rate of patients with systemic lupus erythematosus. Iran J Dermatol 2003;6(4):17-23. [Persian].
- Hoseini SS, Talebi SS, Motaghi Z, Rahmanian V. Predictors of health-related quality of life in women with lupus. J Shahid Sadoughi Univ Med Sci 2022; 30(1):4441-51. doi: 10.18502/ssu.v30i1.9099.[Persian] [Crossref] [ Google Scholar]
- Nasiri N, Golafshan H. Hematologic manifestation of systemic lupus erythematosus. Laboratory & Diagnosis 2021;12(50):15-9. [Persian].
- Brugos B, Kiss E, Dul C, Gubisch W, Szegedi G, Sipka S. Measurement of interleukin-1 receptor antagonist in patients with systemic lupus erythematosus could predict renal manifestation of the disease. Hum Immunol 2010; 71(9):874-7. doi: 10.1016/j.humimm.2010.06.004 [Crossref] [ Google Scholar]
- Umare V, Pradhan V, Nadkar M, Rajadhyaksha A, Patwardhan M, Ghosh KK. Effect of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) on clinical manifestations in Indian SLE patients. Mediators Inflamm 2014; 2014:385297. doi: 10.1155/2014/385297 [Crossref] [ Google Scholar]
- Cigni A, Pileri PV, Faedda R, Gallo P, Sini A, Satta AE. Interleukin 1, interleukin 6, interleukin 10, and tumor necrosis factor α in active and quiescent systemic lupus erythematosus. J Investig Med 2014; 62(5):825-9. doi: 10.2310/jim.0000000000000085 [Crossref] [ Google Scholar]
- Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol 2008; 127(3):385-93. doi: 10.1016/j.clim.2008.01.019 [Crossref] [ Google Scholar]
- Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 2000; 9(8):589-93. doi: 10.1191/096120300678828703 [Crossref] [ Google Scholar]
- Zhao XF, Pan HF, Yuan H, Zhang WH, Li XP, Wang GH. Increased serum interleukin 17 in patients with systemic lupus erythematosus. Mol Biol Rep 2010; 37(1):81-5. doi: 10.1007/s11033-009-9533-3 [Crossref] [ Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008; 28(4):454-67. doi: 10.1016/j.immuni.2008.03.004 [Crossref] [ Google Scholar]
- Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 2009; 60(5):1472-83. doi: 10.1002/art.24499 [Crossref] [ Google Scholar]
- Tang Y, Tao H, Gong Y, Chen F, Li C, Yang X. Changes of serum IL-6, IL-17, and complements in systemic lupus erythematosus patients. J Interferon Cytokine Res 2019; 39(7):410-5. doi: 10.1089/jir.2018.0169 [Crossref] [ Google Scholar]
- Boehme MW, Raeth U, Galle PR, Stremmel W, Scherbaum WA. Serum thrombomodulin-a reliable marker of disease activity in systemic lupus erythematosus (SLE): advantage over established serological parameters to indicate disease activity. Clin Exp Immunol 2000; 119(1):189-95. doi: 10.1046/j.1365-2249.2000.01107.x [Crossref] [ Google Scholar]
- Koca SS, Özgen M, Işık B, Dağlı MN, Üstündağ B, Işık A. Serum salusin-α levels in systemic lupus erythematosus and systemic sclerosis. Eur J Rheumatol 2014; 1(1):14-7. doi: 10.5152/eurjrheum.2014.004 [Crossref] [ Google Scholar]
- Mak A, Tang CS, Ho RC. Serum tumour necrosis factor-alpha is associated with poor health-related quality of life and depressive symptoms in patients with systemic lupus erythematosus. Lupus 2013; 22(3):254-61. doi: 10.1177/0961203312471872 [Crossref] [ Google Scholar]
- Metsärinne KP, Nordström DC, Konttinen YT, Teppo AM, Fyhrquist FY. Plasma interleukin-6 and renin substrate in reactive arthritis, rheumatoid arthritis, and systemic lupus erythematosus. Rheumatol Int 1992; 12(3):93-6. doi: 10.1007/bf00290261 [Crossref] [ Google Scholar]
- Uchida M, Ooka S, Goto Y, Suzuki K, Fujimoto H, Ishimori K. Anti-IL-10 antibody in systemic lupus erythematosus. Open Access Rheumatol 2019; 11:61-5. doi: 10.2147/oarrr.s191953 [Crossref] [ Google Scholar]
- Yao Y, Wang JB, Xin MM, Li H, Liu B, Wang LL. Balance between inflammatory and regulatory cytokines in systemic lupus erythematosus. Genet Mol Res 2016; 15(2):1-8. doi: 10.4238/gmr.15027626 [Crossref] [ Google Scholar]
- Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med 1994; 179(1):305-10. doi: 10.1084/jem.179.1.305 [Crossref] [ Google Scholar]
- Abd Elazeem MI, Mohammed RA, Abdallah NH. Correlation of serum interleukin-10 level with disease activity and severity in systemic lupus erythematosus. Egypt Rheumatol Rehabil 2018; 45(1):25-33. [ Google Scholar]
- Wardowska A, Komorniczak M, Skoniecka A, Bułło-Piontecka B, Lisowska KA, Dębska-Ślizień MA. Alterations in peripheral blood B cells in systemic lupus erythematosus patients with renal insufficiency. Int Immunopharmacol 2020; 83:106451. doi: 10.1016/j.intimp.2020.106451 [Crossref] [ Google Scholar]
- Liu Y, Zhu T, Cai G, Qin Y, Wang W, Tang G. Elevated circulating CD4 + ICOS + Foxp3 + T cells contribute to overproduction of IL-10 and are correlated with disease severity in patients with systemic lupus erythematosus. Lupus 2011; 20(6):620-7. doi: 10.1177/0961203310392431 [Crossref] [ Google Scholar]
- Korganow AS, Knapp AM, Nehme-Schuster H, Soulas-Sprauel P, Poindron V, Pasquali JL. Peripheral B cell abnormalities in patients with systemic lupus erythematosus in quiescent phase: decreased memory B cells and membrane CD19 expression. J Autoimmun 2010; 34(4):426-34. doi: 10.1016/j.jaut.2009.11.002 [Crossref] [ Google Scholar]