Biomed Res Bull. 1(3):109-112.
doi: 10.34172/biomedrb.2023.21
Original Article
Visualized Rapid Brain Extraction in Rats
Faraz Norouzi-Bonab 1  , Kimia Zabihi 1
, Kimia Zabihi 1  , Mahsa Hasanzadeh-Moghadam 2, Seyed Zanyar Athari 3, 4, *
, Mahsa Hasanzadeh-Moghadam 2, Seyed Zanyar Athari 3, 4, * 
Author information:
1Faculty of Pharmacy, Eastern Meditation University, Famagusta, TRNC via Mersin 10, Turkey
2Department of Anatomical Sciences, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
3Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Medical Physiology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Rapid brain extraction is essential for studies involving neural functions, such as electrophysiology and neurochemical experiments. Streamlining the pre-processing pipeline through rapid rat brain extraction improves research by avoiding bias and enhancing efficiency. The manual extraction of brains from rodents is time-consuming, which can affect research outcomes. Achieving fresh brain tissue is essential for experimental procedures in neuroscience, neurophysiology, and neuropharmacology, which are important for evaluating molecular alterations in proteins, mRNAs, and electrophysiology. This study focuses on introducing a rapid and simple technique for extracting a rat’s brain using a rodent skull awl-dilator. It also includes a video demonstrating both the traditional and novel approaches to brain extraction.
Keywords: Brain extraction, Rats, Rodent skull awl-dilator, Neurophysiology
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In neuroscience studies, the rat has a special place due to its physiological and anatomical characteristics, as well as its similarity to humans. Due to ethical restrictions, rats are primarily utilized for investigating the pathophysiology of diseases and drug effects in the initial research phase, as it cannot be directly performed on humans.1,2 The use of immunohistochemistry, polymerase chain reaction analysis, and staining techniques allows for an accurate and comprehensive examination of the brain. Quick and damage-free brain extraction is essential for studies involving brain neurogenesis, histopathological changes, and genetic/protein alterations. Therefore, a controlled, fast, and safe method is highly important.
There are various techniques for extracting the brains of rodents, each with its own advantages and disadvantages.3 Various techniques, ranging from manual to automatic, are employed for brain extraction in rat research. Sedation, removal of skin, muscle, and facia from the skull, and the use of specific tools are part of the manual methods used to extract brain tissue. The potential risks of rat brain extraction include tissue damage, the need for precise dissection to maintain brain tissue integrity, and the requirement for anesthesia and sacrifice procedures to minimize animal distress. Moreover, manual brain extraction methods are prone to being time-consuming, laborious, and operator-dependent, which can result in low reproducibility and potential variability in the outcomes.
Manual rat brain extraction has various advantages. It allows for precise control over the extraction process, can be tailored to the specific characteristics of the brain tissue, and requires no advanced computational resources. However, this method has some disadvantages, such as being time-consuming and labor-intensive, especially with high-resolution datasets, operator-dependent, leading to potential variability in results, and challenging due to the irregular shape of rat brains and ambiguous boundaries.
The rodent skull awl-dilator was introduced to increase the efficiency of the manual method. The tool has a sharp tip that can be opened and closed using two metal handles with a built-in spring. The skull can be split open by creating a hole in the bone between the eyes and squeezing the handles. This study aimed to explore a new method for speeding up brain extraction and its accessibility (Movie, Chapter 1).
Protocol
Equipment and Apparatus
Table 1 provides the equipment and chemical material needed to perform the steps of extracting the rat brain.
Table 1.
List of Equipment and Chemical Material for Brain Extraction
|
Equipment
|
Chemical
|
| Bohler bone cutter |
Alcohol (70%) |
| Cold plate |
Ketamine and xylazine |
| Double edge razor blades |
Normal saline (0.9% saline) |
| Fine scissor |
|
| Forceps, fine tip, and curved |
|
| Laboratory spatula |
|
| Petri dish |
|
| Pointed curved tweezer |
|
| Rodent skull awl-dilator |
|
| Scalpel handle (size 3) |
|
| Small animal decapitator guillotine |
|
| Straight scissors |
|
| Surgical blade (gauges 10 and 15) |
|
| Syringe (1 mL and 3 mL) |
|
Traditional Brain Extraction Procedure
-
Inject ketamine and xylazine (90 mg/kg and 10 mg/kg, respectively) intraperitoneally to produce sedation and deep anesthesia in the male rat.
-
Return the rat to its cage. Then, at intervals of approximately three minutes, check for sedation by assessing the absence of the toe-pinch reflex in the animal. If the rat does not exhibit signs of sedation, an additional half dose of ketamine can be given intraperitoneally.
-
Once the animal is deeply anesthetized and there is a complete lack of reflexes, proceed to insert the rat’s head and the initial portion of its neck into the decapitator. Subsequently, take off the head along with approximately 1 cm of the neck.
-
Using a straight scissor, cut the scalp from the midline from backward forward.
-
Expose each side of the skull by cutting each temporalis muscle on each side with the surgical blade and removing them.
-
Cut the bone between the eyes, the proximal end of the neck, and vertebrae from the occipital bone with a Bohler bone cutter. Remove any remaining parts of muscles attached to the posterior and inferior parts of the skull.
-
Using the Bohler bone cutter, cut the zygomatic arches, coronoid process, and angular process of the mandible on each side.
-
Gently grasp the foramen magnum with a Bohler bone cutter, with caution not to injure the spinal cord and proximally the brain stem. Remove the occipital bone to expose the brain stem and the cerebellum.
-
Using the Bohler bone cutter, cut and extract part of the basal part of the skull, making temporal and parietal bones ready to be removed.
-
The tip of the Bohler bone cutter should be inserted less than 1 mm between the lateral side of the brain and the temporal bone, taking off as much bone as possible while avoiding any pressure on the brain. Repeat that on the other side of the brain.
-
Gently, pull up the parietal bones from the backup and take them off. At this stage, parietal bones will be removed, along with part of the temporal bones as well. Most of the cerebral cortex is now exposed.
-
The frontal bone is tough. Similarly, insert less than 1 mm of the tip of the Bohler bone cutter between the frontal bone superiorly and the cortex of the brain beneath it and start to trim from that frontal bone without creating pressure on the cortex of the brain. Continue until you expose the olfactory bulbs.
-
While the superior surface of the brain is relieved, move back to the inferior surface of the brain and cut the most prominent nerves there, including the trigeminal and optic nerves. At this stage, the brain is still connected to its tip by olfactory bulbs.
-
Using a laboratory spatula, separate the olfactory bulbs, and the brain is free to be extracted (Movie, Chapter 2).4
Novel Brain Extraction Method
-
Inject ketamine and xylazine (90 mg/kg and 10 mg/kg, respectively) intraperitoneally to produce sedation and deep anesthesia in the male rat.
-
Return the rat to its cage. Then, at intervals of approximately three minutes, check for sedation by assessing the absence of the toe-pinch reflex in the animal. If the rat does not exhibit signs of sedation, an additional half dose of ketamine can be given intraperitoneally.
-
Once the animal is deeply anesthetized and there is a complete lack of reflexes, proceed to insert the rat’s head and the initial portion of its neck into the decapitator. Subsequently, take off the head along with approximately 1 cm of the neck.
-
Using a straight scissor, cut the scalp from the midline from backward forward.
-
Expose each side of the skull by cutting each temporalis muscle on each side with the surgical blade and removing them.
-
Cut the bone between the eyes by rodent skull awl-dilator and the proximal end of the neck and vertebrae from the occipital bone by a Bohler bone cutter. Most of the cerebral cortex is now exposed. Remove any remaining parts of muscles attached to the posterior and inferior parts of the skull using a Bohler bone cutter as well.
-
Using the Bohler bone cutter, cut the zygomatic arches, coronoid process, and angular process of the mandible on each side.
-
Using the Bohler bone cutter, cut and extract part of the basal part of the skull, making temporal and parietal bones ready to be removed.
-
While the superior surface of the brain is relieved, move back to the inferior surface of the brain and cut the most prominent nerves there, including the trigeminal and optic nerves. At this stage, the brain is still connected to its tip by olfactory bulbs.
-
Using a laboratory spatula, separate the olfactory bulbs, and the brain is free to be extracted (Movie, Chapter 3) (Table 2).
Table 2.
Company and contreis
|
Equipment
|
|
Chemical
|
|
| Bohler bone cutter |
Aesculap - Germany |
Alcohol (70 %) |
Kimia Alcohol Zanjan - Iran |
| Cold Plate |
Did Sabz - Iran |
Ketamine and Xylazine |
Alfasan - Holland |
| Double edge razor blades |
Lord - Egypt |
Normal saline (0.9 % saline) |
Samen - Iran |
| Fine scissor |
Aesculap - Germany |
|
|
| Forceps, fine tip, curved |
Aesculap - Germany |
|
|
| Laboratory spatula |
Cole Parmer-France |
|
|
| Petri dish |
AHN Biotechnologie GmbH - Germany |
|
|
| Pointed curved tweezer |
Aesculap - Germany |
|
|
| Rodent skull awl-dilator |
Arman Poshtiban Teb - Iran |
|
|
| Scalpel handle (size 3) |
Aesculap - Germany |
|
|
| Small animal decapitator guillotine |
Tajhiz Gostar Omid Iranian - Iran |
|
|
| Straight scissors |
Aesculap - Germany |
|
|
| Surgical blade (gauges 10 and 15) |
Martin - Germany |
|
|
| Syringe (1 ml and 3 ml) |
Soha - Iran |
|
|
Brain Dissection Procedure
Clean the cold plate, the dissecting microscope, and the equipment using 70% ethanol. For solidifying and rigidity purposes, the rat brain, dissection equipment, and stage of dissection should be kept cold by keeping them on the cold plate during the procedure. Then, put a petri dish on the cold plate located on the dissection stage of the microscope.
Anatomical Sites in the Rat Brain
As shown in Figure 1, after extracting the rat brain, it is possible to access the distal (Figure 1A), ventral (Figure 1B), and anatomical regions from the lateral and medial views (Figure 1C). Different cortical lobes, the cerebellum, spinal cord, and other regions are depicted in Figure 1C as important anatomical regions inside the brain (Movie, Chapter 4).
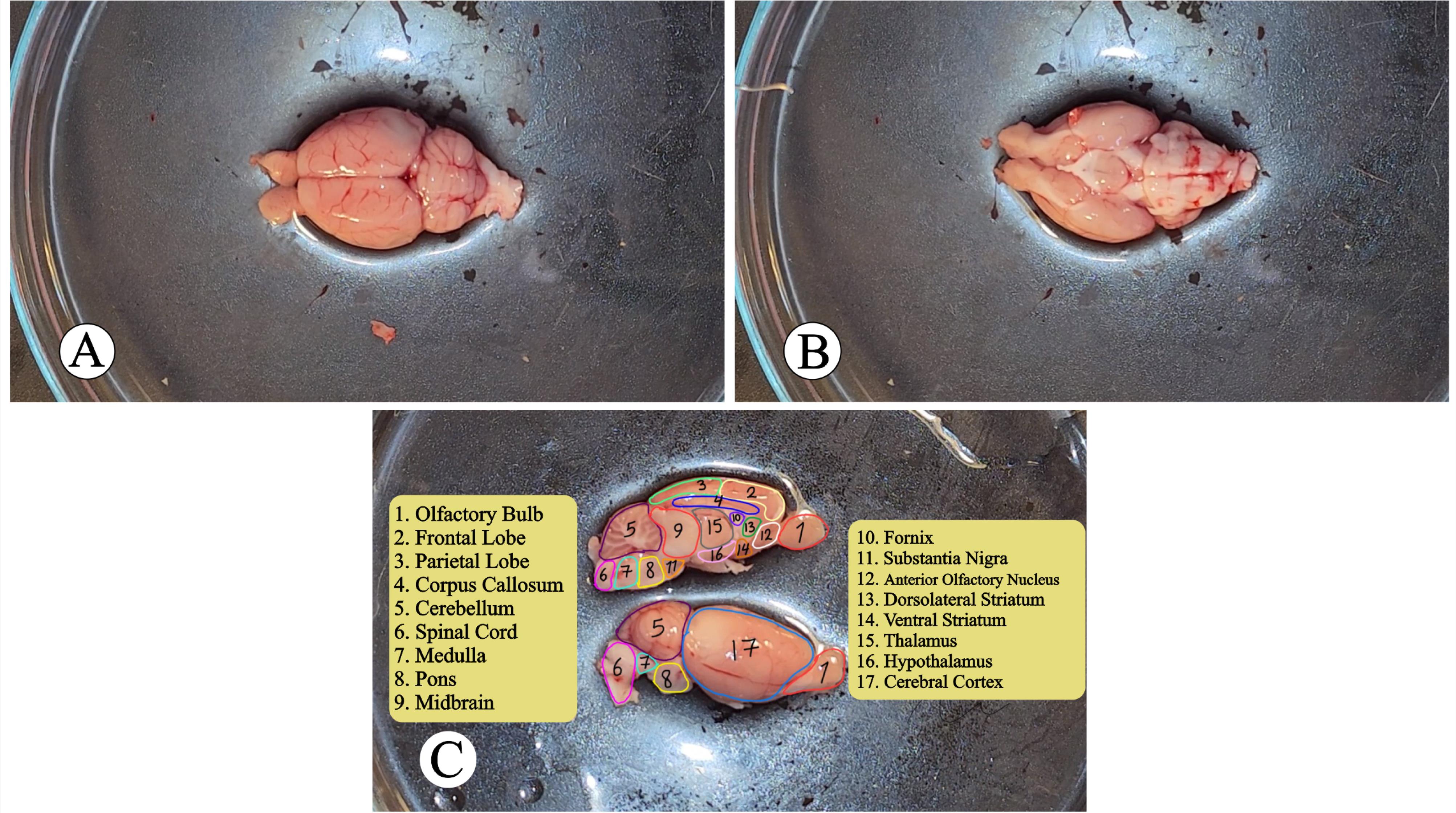
Figure 1.
(A) Dorsal View, (B) Ventral View, and (C) Lateral and Ventral View of Rat Brain
.
(A) Dorsal View, (B) Ventral View, and (C) Lateral and Ventral View of Rat Brain
Conclusion
Based on the results of the studies and the comparison of the mentioned techniques, the novel method is recommended for extracting the brain in rats to study molecular, electrophysiological, biochemical, pharmacological, and histopathological changes to avoid wasting time and the occurrence of desired changes (Movie, Chapter 5).
Authors’ Contribution
Conceptualization: Seyed Zanyar Athari.
Data curation: Faraz Norouzi-Bonab, Mahsa Hasanzadeh-Moghadam, Seyed Zanyar Athari.
Formal analysis: Faraz Norouzi-Bonab, Kimia Zabihi.
Funding acquisition: Seyed Zanyar Athari.
Investigation: Faraz Norouzi-Bonab, Seyed Zanyar Athari.
Methodology: Mahsa Hasanzadeh-Moghadam, Seyed Zanyar Athari.
Project administration: Seyed Zanyar Athari.
Resources: Mahsa Hasanzadeh-Moghadam, Seyed Zanyar Athari.
Software: Faraz Norouzi-Bonab, Kimia Zabihi.
Supervision: Seyed Zanyar Athari.
Validation: Seyed Zanyar Athari.
Visualization: Faraz Norouzi-Bonab, Mahsa Hasanzadeh-Moghadam, Mahsa Hasanzadeh-Moghadam.
Writing–original draft: Faraz Norouzi-Bonab, Kimia Zabihi.
Writing–review & editing: Seyed Zanyar Athari.
Competing Interests
All of the authors declare that they have no conflict of interests.
Ethical Approval
This study was approved by Research Ethics Committee of Vice-Chancellor in Research Affairs - Tabriz University of Medical Sciences (Code: IR.TBZMED.VCR.REC.1397.053).
Funding
None.
Supplementary Files
Movie: Rapid Brain Extraction in Rats
(mp4)
References
- Mukherjee P, Roy S, Ghosh D, Nandi SK. Role of animal models in biomedical research: a review. Lab Anim Res 2022; 38(1):18. doi: 10.1186/s42826-022-00128-1 [Crossref] [ Google Scholar]
- Papadimitriou D, Xanthos T, Dontas I, Lelovas P, Perrea D. The use of mice and rats as animal models for cardiopulmonary resuscitation research. Lab Anim 2008; 42(3):265-76. doi: 10.1258/la.2007.006035 [Crossref] [ Google Scholar]
- Liu Y, Unsal HS, Tao Y, Zhang N. Automatic brain extraction for rodent MRI images. Neuroinformatics 2020; 18(3):395-406. doi: 10.1007/s12021-020-09453-z [Crossref] [ Google Scholar]
- Aboghazleh R, Boyajian SD, Atiyat A, Udwan M, Al-Helalat M, Al-Rashaideh R. Rodent brain extraction and dissection: a comprehensive approach. MethodsX 2024; 12:102516. doi: 10.1016/j.mex.2023.102516 [Crossref] [ Google Scholar]