Biomed Res Bull. 1(4):135-140.
doi: 10.34172/biomedrb.2023.26
Original Article
Combination of Vitamin D Intake and Aerobic Exercise Protected Young Female Rats From Oxidative Stress and Memory Impairment Caused by Maternal Vitamin D Deficiency
Faraz Norouzi Bonab 1  , Kimia Zabihi 1, Seyed Zanyar Athari 2, 3, *
, Kimia Zabihi 1, Seyed Zanyar Athari 2, 3, * 
Author information:
1Faculty of Pharmacy, Eastern Meditation University, Famagusta, TRNC via Mersin 10, Turkey
2Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Medical Physiology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Vitamin D deficiency (VDD) is a crucial global health issue that causes increased oxidative stress (OS) and memory disorders. This study aimed to evaluate the effects of vitamin D (VD) and aerobic exercise (EXE) combination therapy on OS and memory impairment caused by maternal VDD in young female rats.
Methods:
Forty female Wistar rats, aged 3 weeks and weighing 70±5 g, were divided into five groups. The Sham with a standard diet (SSD) group received a standard diet (SD) 6 weeks before mating until the end of lactation, and the VDD group consumed VDD a week before mating until the last day of lactation. The VDD+VD group received SD containing 1,000 IU/kg VD a week before mating until the end of lactation, and the VDD+EXE group received SD one week before mating until the last day of gestation and underwent treadmill exercise from a week before mating until gestational day 20. The last group received VDD+VD+EXE. Finally, spatial memory was assessed, and the animals were sacrificed on post-natal day 40. Left hippocampal OS status was evaluated using the enzyme-linked immunosorbent assay.
Results:
Maternal VDD caused an elevation in hippocampal OS along with memory impairment (P < 0.05). Vitamin D supplementation and aerobic exercise, alone or in combination, improved memory performance in the T-maze and novel object recognition test tasks by reducing lipid peroxidation levels and increasing enzymatic antioxidant activity in the hippocampus (P < 0.05).
Conclusion:
The findings indicated that vitamin D supplementation and aerobic exercise can effectively reduce the amount of OS and thus improve the level of memory in rats.
Keywords: Aerobic exercise, Oxidative stress, Memory, Vitamin D deficiency
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Vitamin D deficiency (VDD) is a prevalent global health issue, with approximately 12% of the population globally affected by this condition and 50% of the people having vitamin D insufficiency.1 High-risk groups for VDD are those with limited sunlight exposure or dark skin, inadequate intake of vitamin D-rich foods, the elderly, nursing home residents, obese individuals, and hospitalized patients.2 Despite attempts to combat this issue, recent studies have revealed that VDD and insufficiency are still connected to numerous pathological conditions in individuals of all age groups.3,4
VDD has been associated with memory disorders, cognitive decline, and an increased risk of conditions such as depression, Alzheimer’s disease, and other dementias.5 In addition, low levels of vitamin D have been linked to muscle weakness, bone pain, and incorrect growth patterns, particularly in cases of severe deficiency, such as rickets in children.6 Likewise, there is a connection between insufficient vitamin D during pregnancy and abnormal brain morphology and function in offspring, resulting in neurodevelopmental or neurocognitive disorders.7 Therefore, optimal prenatal vitamin D levels are crucial for healthy development and mental health outcomes, as the developing fetus’s entirely relies on the mother’s vitamin D stores.8
Vitamin D can affect memory performance, and its deficiency deteriorates cognitive function.3 Studies have demonstrated that supplementation with vitamin D3 can prevent stress-induced memory deficits in rats by enhancing brain-derived neurotrophic factor (BDNF) levels and reducing oxidative stress (OS) damage.7 Furthermore, vitamin D has a neuroprotective effect through the reduction of OS markers, modulation of calcium homeostasis, and inhibition of inflammatory processes.9 Additionally, vitamin D has the ability to reduce OS through the silent information regulator transcript-1/nuclear-factor erythroid 2–related factor-2/nuclear factor- κBsignaling pathway, leading to the restoration of neuronal synapse protein levels.10
An additional way to boost memory is through aerobic exercise. Studies have demonstrated its ability to prevent memory deficits and reduce brain oxidative damage caused by stressors such as maternal deprivation.11 Moreover, exercise improves the antioxidant response, lowers OS related to aging, and decreases pro-inflammatory signals, ultimately supporting the activation of antioxidant enzymes and increasing nitric oxide availability.12,13 Furthermore, exercise training could enhance BDNF and decrease oxidative damage markers, including malondialdehyde (MDA) and protein carbonyl concentration, thus lessening OS and supporting neuronal function.14 Further, gestational exercise has a positive impact on the developing fetus’s health and improves cognitive function in offspring by enhancing hippocampal BDNF levels and neurogenesis.15,16
This study aimed to assess the combined impact of vitamin D intake and aerobic exercise on memory function and hippocampal OS in female offspring rats born to vitamin D-deficient mothers.
Materials and Methods
Animals
Forty female Wistar rats, aged 3 weeks and weighing 70 ± 5 g, were obtained from the Pasteur Institute (Tehran, Iran). They were housed in groups of three per cage under controlled conditions, namely, a constant temperature of 21 ± 2 ℃ and a 12-hour light/dark cycle (lights on from 7:00 am to 7:00 pm), and food and water ad libitum.
Study Design
Following a week of acclimating to the new housing, the rats were unintentionally divided into the Sham with a standard diet (n = 8) and VDD (n = 40) groups. The Sham group of rats was given a standard diet with 1000 IU/kg vitamin D3, while the VDD group received a VDD diet with 0 IU/kg vitamin D3 (Royan Research Institute of Isfahan, Iran) for 6 weeks.17
After receiving VDD or SD for six weeks, blood samples were taken from the tail vein to confirm VDD, which was indicated by serum 25(OH)D levels < 10 ng/mL. Accordingly, the animals underwent several interventions. In the SSD group, SD was continued from 6 weeks before mating until the end of lactation. In group two, VDD was continued from a week before mating until the last day of lactation. The VDD + VD group received SD containing 1000 IU/kg vitamin D a week before mating until the end of lactation, and the VDD + EXE group received SD one week before mating until the last day of gestation and underwent treadmill exercise from a week before mating until gestational day 20. Finally, the VDD + VD + EXE group was given SD a week before mating until the end of lactation and engaged in treadmill exercise from a week before mating until gestational day 20. Female offspring were weaned at the end of lactation (PND 21) and divided into six groups based on their maternal arrangements. They were then kept under standard laboratory conditions and provided with SD and water ad libitum until PND 40.
Exercise Protocol
Animals in the VDD + EXE and VDD + VD + EXE groups underwent daily physical exercise on a treadmill, without incline, from pre-mating until gestational day 20. The warm-up session of the exercise protocol began with a 3-minute duration and a speed of 8 m/minute. They were encouraged to run by applying electric shocks (1 mA). The speed and running time were incrementally increased each day, starting from 10 m/min for 3 minutes in the initial sessions and reaching a speed of 12 m/minute for 30 minutes on the last day.15,18 The non-exercise groups were brought to the exercise room and placed on a deactivated treadmill, using the same methods as the exercise groups. All dams at gestational day 21 were kept at rest to deliver naturally.
Behavioral Tests
All behavioral assessments were performed in the light phase in a room separate from the housing room in a blinding manner. The animals were transferred to the testing room 30 minutes prior to the test in order to adapt to the behavioral room. The Noldus EthoVisionTM video tracking software (Noldus, The Netherlands) was utilized to assess behavioral activities.
T-maze
The T-maze box had a start arm, a left arm, and a right arm (width 12 cm, height 20 cm, start arm 76 cm, right and left arms both 31.5 cm). On day one, the mice were situated at the start of the T-maze box, with the right arm blocked, and allowed to freely explore for 10 minutes, and the frequency of left arm entries was recorded. After one day, the door that was blocking the path was removed, and the rats were put back in the same position. Then, the number of entries into the familiar and new paths was measured for 10 minutes. The percentage of the left and right arm inputs was used to calculate spatial perception ability.
Novel Object Recognition Test
The Novel Object Recognition Test (NORT) is a simple learning test with the least motivational component, which causes the animal’s natural tendency to spend more time exploring new objects than old objects. The test device was a Plexiglas open field box with dimensions of 40 × 50 × 50, which was placed in a soundproof room. In this test, the objects have different shapes but equal complexity. Placing the nose at a distance of 2 cm from the object defined the exploratory behavior. Prior to each trial, the field and objects were cleaned using 70% alcohol to eliminate any scent marks.
The test was performed in three consecutive trials. During the first day (the habituation phase), the animals were introduced to an empty chamber for 10 minutes to become accustomed to the new surroundings, and their locomotor activity was measured. The second trial (the training phase) was conducted twenty-four hours after the first trial. Two similar objects (A1 and A2) were placed inside the box, and the animal was given approximately 10 minutes to explore them. After an hour, the animal was returned to the box, with one of the familiar objects exchanged for a new object (a novel one), and given 10 minutes to freely explore the objects.
Sampling
On PND 40, the animals were fully anesthetized using a combination of ketamine and xylazine (90 and 10 mg/kg, respectively). The rats were sacrificed by decapitation, and the entire brain was carefully removed from the skull. The left and right hippocampal regions were quickly dissected on ice and stored at -70 ℃.
Left Hippocampal Oxidative Stress Status
The left hippocampus samples were homogenized in a 1.15% KCl solution and then centrifuged at 12 000 rpm for 15 minutes at 4°C to obtain the supernatant. The protein concentration in the supernatant was estimated using the Bradford method.
Malondialdehyde Levels
The concentration of MDA, a marker for lipid peroxidation, was evaluated using the thiobarbituric acid reaction colorimetric assay. The supernatant (200 μL) was briefly mixed with thiobarbituric acid (1 mL), trichloroacetic acid, and Tris-HCl (0.9 mL, pH = 7.4). The mixture was then incubated at 100°C for 20 minutes. After centrifuging the samples at 3000 rpm for 10 minutes, the optical density of the supernatant was measured at 540 nm using a plate reader, and the results were reported as nmol/mg protein.19
Glutathione Peroxidase Activity
The enzyme activity of glutathione peroxidase (GPx) was measured spectrophotometrically using the RANSEL kit from Randox Laboratories Ltd., according to Paglia and Valentine’s method. At 37 °C, the absorbance was measured at 340 nm, and the outcomes were reported as nmol/mg protein.20
Catalase Activity
The activity of catalase was determined using the method developed by Aebi; in brief, 30 mM hydrogen peroxide (H2O2) was used as a substrate, and 50 mM phosphate buffer (pH = 7) was utilized as an alternative substrate in the blank. By adding H2O2, the reaction was initiated, and the absorption at 240 nm was measured for 3 minutes.21
Statistical Analysis
The data are shown as means ± standard deviations (SD). The data were statistically analyzed using GraphPad Prism 9. Between-group comparisons were made using a one-way analysis of variance (ANOVA) and Tukey’s post-hoc test. The statistical significance was established with a P value < 0.05.
Results
Confirmation of the Vitamin D Deficiency Model
According to the results, VDD for 6 weeks led to a significant decrease in serum 25-(OH) D levels in VDD rats compared to SSD rats on a normal vitamin D diet (P < 0.001, Figure 1).
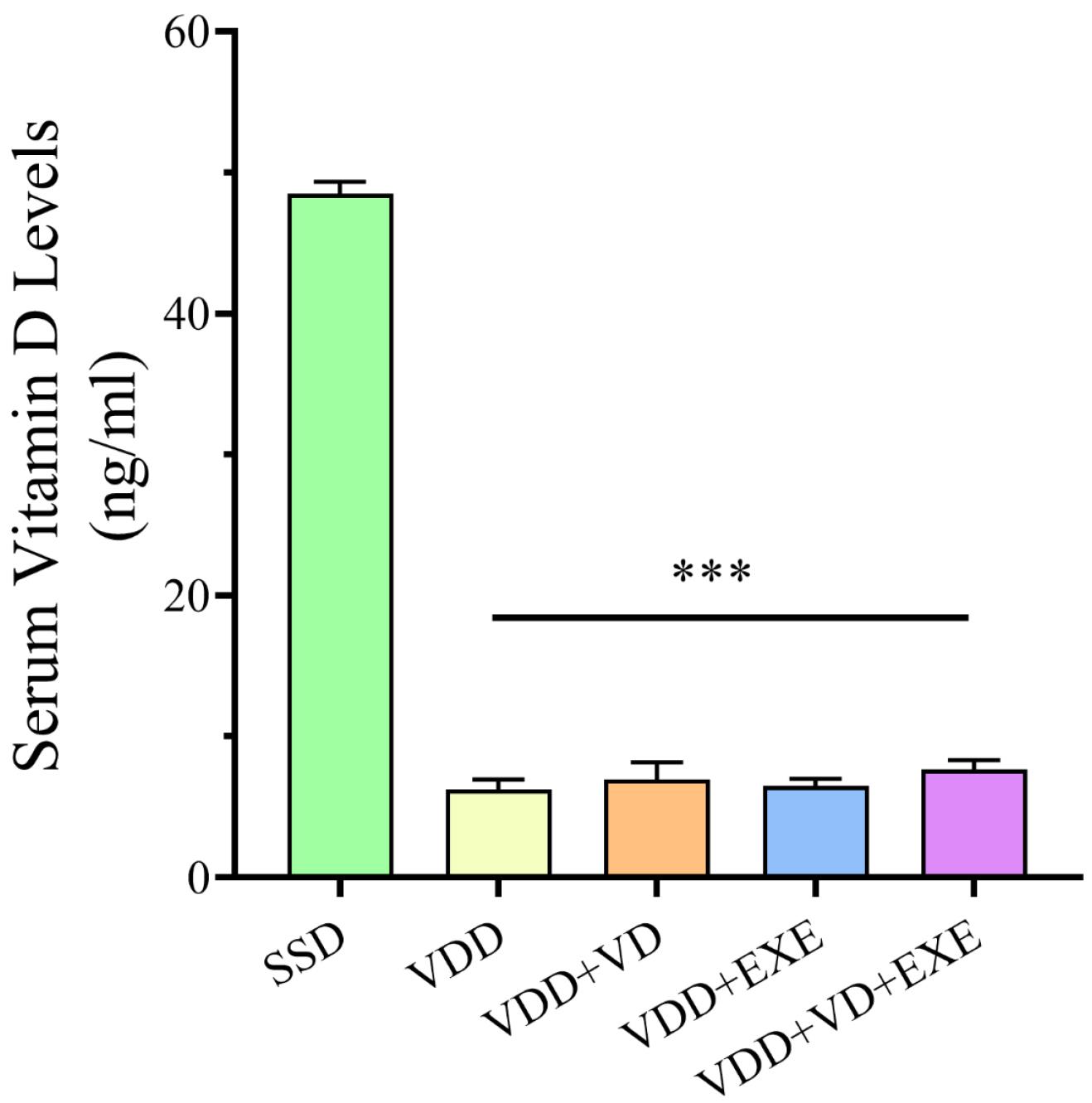
Figure 1.
Maternal Serum Vitamin D Levels (ng/mL) in the Experimental Animals at the End of 6 Weeks. Note. Data are presented as means ± standard deviations (n = 6). ***P < 0.001 compared to the SSD group. SSD: Sham with a standard diet; VDD: Vitamin D deficiency
.
Maternal Serum Vitamin D Levels (ng/mL) in the Experimental Animals at the End of 6 Weeks. Note. Data are presented as means ± standard deviations (n = 6). ***P < 0.001 compared to the SSD group. SSD: Sham with a standard diet; VDD: Vitamin D deficiency
Memory Performance
As shown in Figure 2A, in all groups, there was no significant difference in the exploration time of the two identical objects (A1 and A2) in the second phase of NORT. In the VDD group, the exploration time for the novel object was lower than the familiar one (P < 0.001, Figure 2B). On the contrary, the SSD and VDD + VD + EXE groups demonstrated longer exploration times for novel objects than familiar objects (P < 0.001). Moreover, the results of the recognition index (RI) showed that the VDD group had a lower RI than the SSD group (P < 0.001, Figure 2C). In addition, SD (P < 0.01), treadmill exercise (P < 0.01), and combination therapy (P < 0.001) significantly improved RI in the female offspring of the VDD dams.
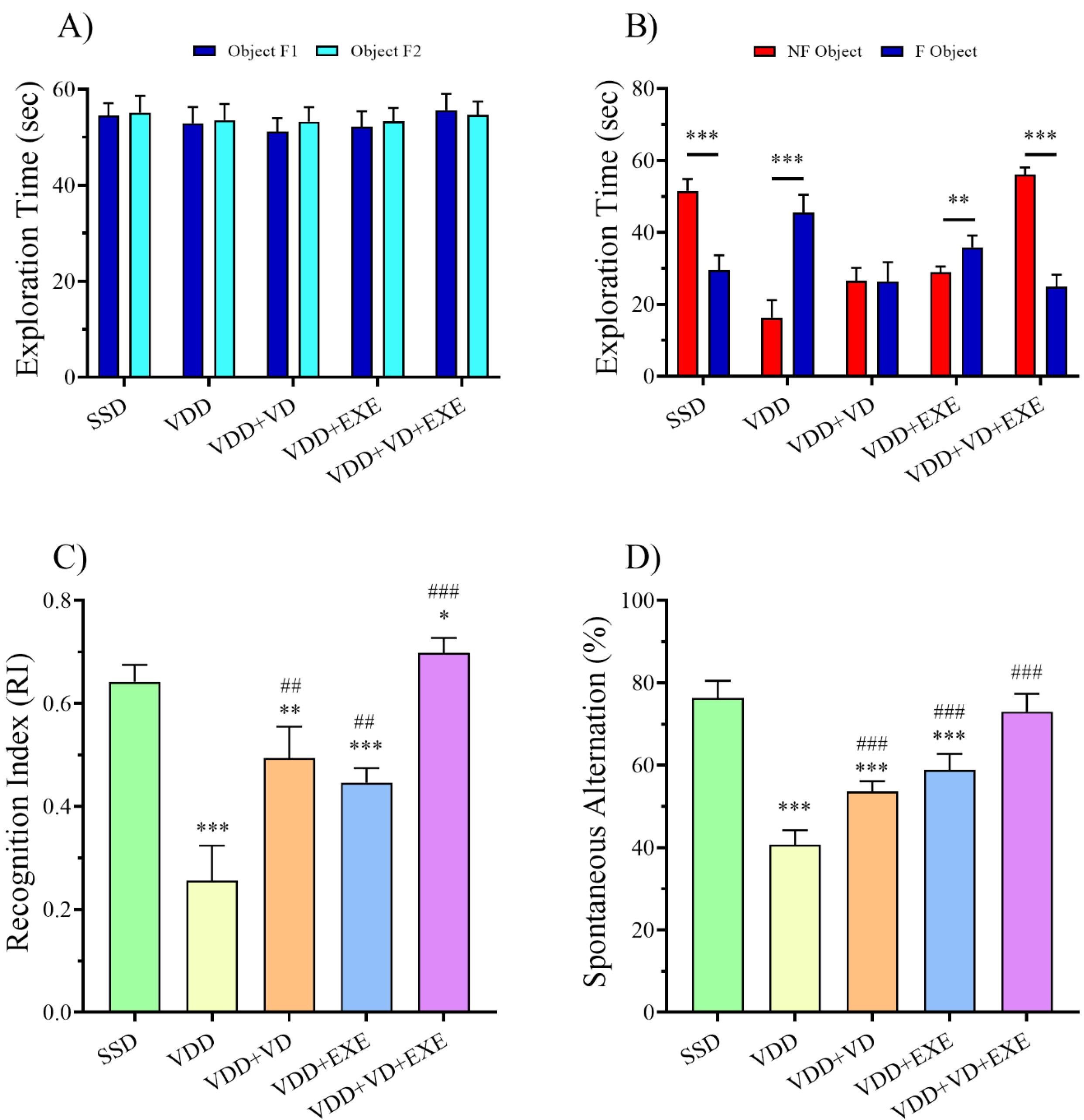
Figure 2.
Effects of VD and EXE, Alone or in Combination, on (A) Exploration Time of Two Similar Objects, (B) Exploration Time of the Familiar or Novel Objects, (C) Recognition Index in the NORT, and (D) Spontaneous Alteration (%) in the T-maze Test. Note. Data are presented as means ± standard deviations (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the SSD group.##P < 0.01, ###P < 0.001 compared to the VDD group. SSD: Sham with a standard diet; VDD: Vitamin D deficiency; VD: Vitamin D; EXE: Exercise
.
Effects of VD and EXE, Alone or in Combination, on (A) Exploration Time of Two Similar Objects, (B) Exploration Time of the Familiar or Novel Objects, (C) Recognition Index in the NORT, and (D) Spontaneous Alteration (%) in the T-maze Test. Note. Data are presented as means ± standard deviations (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the SSD group.##P < 0.01, ###P < 0.001 compared to the VDD group. SSD: Sham with a standard diet; VDD: Vitamin D deficiency; VD: Vitamin D; EXE: Exercise
In the T-maze test, a significant decrease was found in spontaneous alternation percentage in the VDD group as compared to the SDD offspring (P < 0.001, Figure 2D). However, SD (P < 0.001), treadmill exercise (P < 0.001), and combination therapy (P < 0.001) could significantly increase spontaneous alternation percentage compared to the VDD group.
Hippocampal Oxidative Stress Status
Based on the results of one-way ANOVA, the hippocampal GPx and superoxide dismutase (SOD) activities in the VDD group were significantly lower than the SSD group (P < 0.001 for both, Figure 3A and B). However, MDA levels were significantly higher in the VDD group compared to the SSD group (P < 0.001, Figure 3C). On the other hand, supplementation with SD, treadmill exercise, or a combination of SD and EXE markedly (P < 0.001) decreased MDA levels while increasing GPx and SOD activities in the hippocampus of the female offspring of VDD dams.
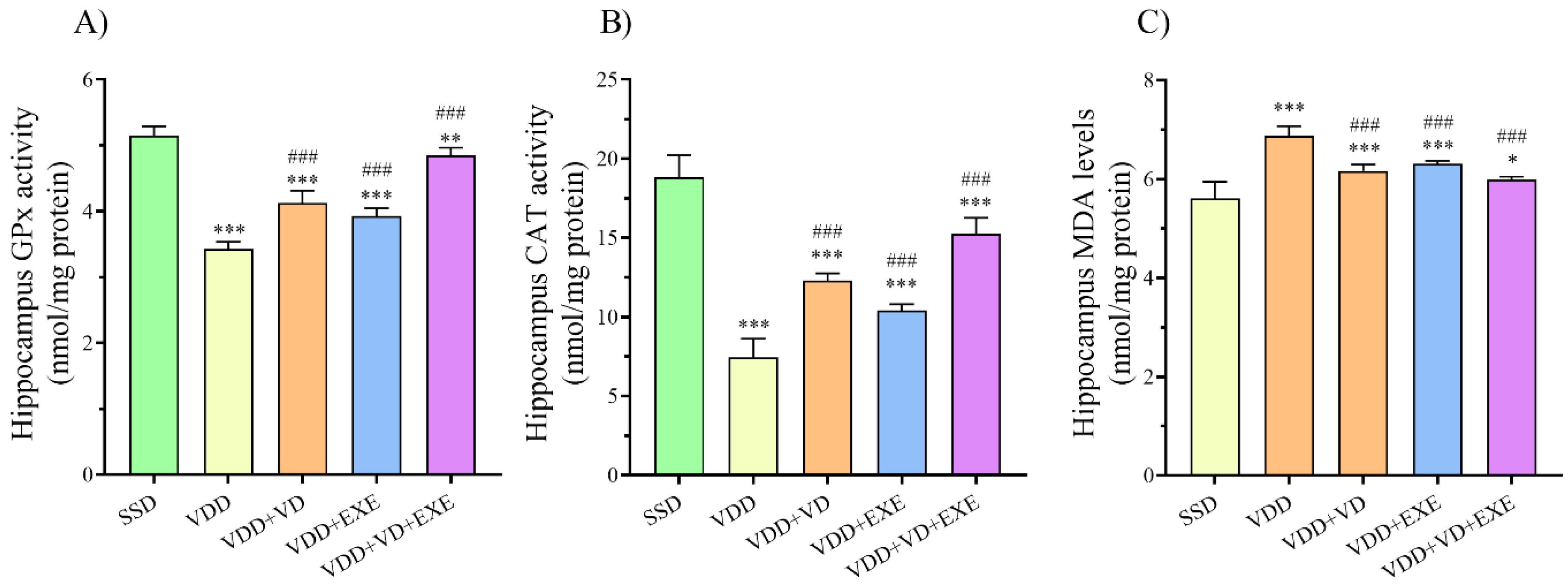
Figure 3.
Effects of VD and EXE, Alone or in Combination, on (A) Hippocampus GPx Activity (nmol/mg Protein), (B) Hippocampus CAT Activity (nmol/mg Protein), and (C) Hippocampus MDA Levels (nmol/mg Protein) in Experimental Animals. Note. Data are presented as means ± standard deviations (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the SSD group. ###P < 0.001 compared to the VDD group. SSD: Sham with standard diet; VDD: Vitamin D deficiency; VD: Vitamin D; EXE: Exercise; GPx: Glutathione peroxidase; CAT: Catalase; MDA: Malondialdehyde
.
Effects of VD and EXE, Alone or in Combination, on (A) Hippocampus GPx Activity (nmol/mg Protein), (B) Hippocampus CAT Activity (nmol/mg Protein), and (C) Hippocampus MDA Levels (nmol/mg Protein) in Experimental Animals. Note. Data are presented as means ± standard deviations (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the SSD group. ###P < 0.001 compared to the VDD group. SSD: Sham with standard diet; VDD: Vitamin D deficiency; VD: Vitamin D; EXE: Exercise; GPx: Glutathione peroxidase; CAT: Catalase; MDA: Malondialdehyde
Discussion
The findings of the present study demonstrated that maternal VDD caused an elevation in hippocampal OS along with memory impairment in young female offspring. On the other hand, vitamin D supplementation and aerobic exercise, alone or in combination, improved memory performance in the T-maze and NORT tasks by reducing lipid peroxidation (malondialdehyde) levels and increasing enzymatic antioxidant activity in the hippocampus.
Evidence suggests that the offspring of mothers with VDD may have learning difficulties and show alterations in brain structures such as the hippocampus. Moreover, there is a connection between maternal VDD and elevated OS in the fetal brain, potentially influencing neurodevelopment. Furthermore, animal studies have shown that vitamin D3 administration can prevent memory deficits and modify biochemical parameters influenced by OS.9,22
Vitamin D, as observed in the present study, is a powerful antioxidant that protects against OS-related damage, such as protein oxidation, lipid peroxidation, and DNA damage.23 Additionally, the findings confirmed an increase in lipid peroxidation in the VDD group, which is in line with earlier research findings.3,24 Furthermore, evidence indicates that vitamin D supplementation can lower OS and enhance the activity of antioxidant enzymes, resulting in a decrease in oxidative/nitrosative stress and inflammation.25 Similar to previous research,24 in this study, it was found that a lack of vitamin D resulted in a reduction in GPx activity, while vitamin D treatment decreased OS by enhancing GSH formation. Proper vitamin D levels regulate intracellular OS-related activities, whereas inadequate levels are ineffective in controlling OS conditions, resulting in amplified intracellular oxidative damage.3 Therefore, it seems that maternal vitamin D replacement protects the hippocampus of their females by suppressing oxidative damage.
Aerobic exercise is another way to improve memory and reduce OS. Although intense exercise can cause muscle oxidative damage, regular exercise boosts antioxidant activities, reducing free radical production and oxidative damage.13 Regular, moderate exercise can help decrease OS and provide various health benefits by strengthening the antioxidant defense system. Exercise is essential for boosting the total antioxidant capacity, thus supporting the idea of exercise-induced hormesis.26 The results of the present study indicated that aerobic exercise results in higher antioxidant enzyme activity and lower lipid peroxidation in the hippocampus of female offspring. Interestingly, the combination therapy of vitamin D and aerobic exercise exhibited greater efficacy than either therapy alone, resulting in improved antioxidant activity.
Memory impairment is a crucial side effect of VDD, resulting from its impact on OS, epigenetics, and gene regulation.27 Optimal vitamin D levels are connected to decreased OS, enhanced mitochondrial and endocrine functions, and a decreased probability of OS-related disorders, including memory disorders.3,28 Research suggests that there is a link between low vitamin D levels and a higher risk of dementia, cognitive decline, and memory loss. Impaired episodic memory and executive dysfunction in adults have been associated with VDD.28,29 According to the results of the present study, VDD caused a decline in memory in female offspring as assessed by NORT and T-maze tests. Vitamin D supplements can prevent memory deficits and enhance synaptic function in the hippocampus of aging rats.30 Additionally, administering vitamin D has been linked to preventing stress-induced memory impairments in animal models.31 Additionally, physical activity induces physiological changes that promote the production of growth factors and enhance the health of new neural cells.32 Furthermore, exercise indirectly enhances memory and cognition by improving mood and sleep while reducing stress and anxiety. Studies have shown that regular physical activity can decrease the tendency for cognitive decline, such as dementia.33
Conclusion
The findings revealed that VDD causes a memory deficit through increasing hippocampus OS. However, vitamin D supplementation along with aerobic exercise can effectively reduce the amount of OS and thus improve the level of memory.
Acknowledgments
The authors would like to thank the scientific guidance of Dr. Fereshte Farajdokht.
Authors’ Contribution
Conceptualization: Seyed Zanyar Athari.
Data curation: Faraz Norouzi Bonab, Seyed Zanyar Athari.
Formal analysis: Faraz Norouzi Bonab, Seyed Zanyar Athari.
Methodology: Seyed Zanyar Athari.
Project administration: Seyed Zanyar Athari.
Supervision: Seyed Zanyar Athari.
Validation: Kimia Zabihi, Seyed Zanyar Athari.
Visualization: Kimia Zabihi, Seyed Zanyar Athari.
Writing–original draft: Faraz Norouzi Bonab, Seyed Zanyar Athari.
Writing–review & editing: Kimia Zabihi, Seyed Zanyar Athari.
Competing Interests
None declared.
Ethical Approval
The procedures followed the protocols approved by the Ethics Committee of Tabriz University of Medical Sciences (Approval No. IR.TBZMED.VCR.REC.1399.180) and the guidelines of the National Institutes of Health.
Funding
None.
References
- Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem?. J Steroid Biochem Mol Biol 2014; 144 Pt A:138-45. doi: 10.1016/j.jsbmb.2013.11.003 [Crossref] [ Google Scholar]
- LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R. Screening for vitamin D deficiency: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(2):109-22. doi: 10.7326/m14-1659 [Crossref] [ Google Scholar]
- Wimalawansa SJ. Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. Biology (Basel) 2019; 8(2):30. doi: 10.3390/biology8020030 [Crossref] [ Google Scholar]
- Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician 2009; 80(8):841-6. [ Google Scholar]
- Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med 2010; 170(13):1135-41. doi: 10.1001/archinternmed.2010.173 [Crossref] [ Google Scholar]
- Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013; 5(1):111-48. doi: 10.3390/nu5010111 [Crossref] [ Google Scholar]
- Eyles DW, Feron F, Cui X, Kesby JP, Harms LH, Ko P. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology 2009; 34 Suppl 1:S247-57. doi: 10.1016/j.psyneuen.2009.04.015 [Crossref] [ Google Scholar]
- Basile LA, Taylor SN, Wagner CL, Quinones L, Hollis BW. Neonatal vitamin D status at birth at latitude 32 degrees 72’: evidence of deficiency. J Perinatol 2007; 27(9):568-71. doi: 10.1038/sj.jp.7211796 [Crossref] [ Google Scholar]
- Gáll Z, Székely O. Role of vitamin D in cognitive dysfunction: new molecular concepts and discrepancies between animal and human findings. Nutrients 2021; 13(11):3672. doi: 10.3390/nu13113672 [Crossref] [ Google Scholar]
- Wang W, Li Y, Meng X. Vitamin D and neurodegenerative diseases. Heliyon 2023; 9(1):e12877. doi: 10.1016/j.heliyon.2023.e12877 [Crossref] [ Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 2008; 88(4):1243-76. doi: 10.1152/physrev.00031.2007 [Crossref] [ Google Scholar]
- El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, Rodríguez-Mañas L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci 2022; 23(15):8713. doi: 10.3390/ijms23158713 [Crossref] [ Google Scholar]
- Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018; 9(24):17181-98. doi: 10.18632/oncotarget.24729 [Crossref] [ Google Scholar]
- Fisher E, Wood SJ, Elsworthy RJ, Upthegrove R, Aldred S. Exercise as a protective mechanism against the negative effects of oxidative stress in first-episode psychosis: a biomarker-led study. Transl Psychiatry 2020; 10(1):254. doi: 10.1038/s41398-020-00927-x [Crossref] [ Google Scholar]
- Gomes da Silva S, de Almeida AA, Fernandes J, Lopim GM, Cabral FR, Scerni DA. Maternal exercise during pregnancy increases BDNF levels and cell numbers in the hippocampal formation but not in the cerebral cortex of adult rat offspring. PLoS One 2016; 11(1):e0147200. doi: 10.1371/journal.pone.0147200 [Crossref] [ Google Scholar]
- Kim TW, Park SS, Park HS. Effects of exercise training during advanced maternal age on the cognitive function of offspring. Int J Mol Sci 2022; 23(10):5517. doi: 10.3390/ijms23105517 [Crossref] [ Google Scholar]
- Kazemi F, Babri S, Keyhanmehr P, Farid-Habibi M, Nayebi Rad S, Farajdokht F. Maternal vitamin D supplementation and treadmill exercise attenuated vitamin D deficiency-induced anxiety-and depressive-like behaviors in adult male offspring rats. Nutr Neurosci 2023; 26(6):470-82. doi: 10.1080/1028415x.2022.2059203 [Crossref] [ Google Scholar]
- Arida RM, Scorza FA, Gomes da Silva S, Cysneiros RM, Cavalheiro EA. Exercise paradigms to study brain injury recovery in rodents. Am J Phys Med Rehabil 2011; 90(6):452-65. doi: 10.1097/PHM.0b013e3182063a9c [Crossref] [ Google Scholar]
- Aguilar Diaz De Leon J, Borges CR. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J Vis Exp. 2020(159):e61122. 10.3791/61122.
- Ghyasi R, Sepehri G, Mohammadi M, Badalzadeh R, Ghyasi A. Effect of mebudipine on oxidative stress and lipid peroxidation in myocardial ischemic-reperfusion injury in male rat. J Res Med Sci 2012; 17(12):1150-5. [ Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol 1984; 105:121-6. doi: 10.1016/s0076-6879(84)05016-3 [Crossref] [ Google Scholar]
- Wang YQ, Geng XP, Wang MW, Wang HQ, Zhang C, He X. Vitamin D deficiency exacerbates hepatic oxidative stress and inflammation during acetaminophen-induced acute liver injury in mice. Int Immunopharmacol 2021; 97:107716. doi: 10.1016/j.intimp.2021.107716 [Crossref] [ Google Scholar]
- Alloubani A, Akhu-Zaheya L, Samara R, Abdulhafiz I, Saleh A, Altowijri A. Relationship between vitamin D deficiency, diabetes, and obesity. Diabetes Metab Syndr 2019; 13(2):1457-61. doi: 10.1016/j.dsx.2019.02.021 [Crossref] [ Google Scholar]
- Câmara AB, Brandão IA. The relationship between vitamin D deficiency and oxidative stress can be independent of age and gender. Int J Vitam Nutr Res 2021; 91(1-2):108-23. doi: 10.1024/0300-9831/a000614 [Crossref] [ Google Scholar]
- Omar HS, Taha FM, Fouad S, Ibrahim FA, El Gendy A, Bassyouni IH. The association between vitamin D levels and oxidative stress markers in Egyptian Behcet’s disease patients. Orphanet J Rare Dis 2022; 17(1):264. doi: 10.1186/s13023-022-02416-4 [Crossref] [ Google Scholar]
- Koyama K. Exercise-induced oxidative stress: a tool for “hormesis” and “adaptive response”. J Phys Fit Sports Med 2014; 3(1):115-20. doi: 10.7600/jpfsm.3.115 [Crossref] [ Google Scholar]
- Singh P, Barman B, Thakur MK. Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front Aging Neurosci 2022; 14:944697. doi: 10.3389/fnagi.2022.944697 [Crossref] [ Google Scholar]
- Goodwill AM, Szoeke C. A systematic review and meta-analysis of the effect of low vitamin D on cognition. J Am Geriatr Soc 2017; 65(10):2161-8. doi: 10.1111/jgs.15012 [Crossref] [ Google Scholar]
- Sultan S, Taimuri U, Basnan SA, Ai-Orabi WK, Awadallah A, Almowald F. Low vitamin D and its association with cognitive impairment and dementia. J Aging Res 2020; 2020:6097820. doi: 10.1155/2020/6097820 [Crossref] [ Google Scholar]
- Bakhtiari-Dovvombaygi H, Izadi S, Zare M, Asgari Hassanlouei E, Dinpanah H, Ahmadi-Soleimani SM. Vitamin D3 administration prevents memory deficit and alteration of biochemical parameters induced by unpredictable chronic mild stress in rats. Sci Rep 2021; 11(1):16271. doi: 10.1038/s41598-021-95850-6 [Crossref] [ Google Scholar]
- Latimer CS, Brewer LD, Searcy JL, Chen KC, Popović J, Kraner SD. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci U S A 2014; 111(41):E4359-66. doi: 10.1073/pnas.1404477111 [Crossref] [ Google Scholar]
- Di Liegro CM, Schiera G, Proia P, Di Liegro I. Physical activity and brain health. Genes (Basel) 2019; 10(9):720. doi: 10.3390/genes10090720 [Crossref] [ Google Scholar]
- Rashid MH, Zahid MF, Zain S, Kabir A, Hassan SU. The neuroprotective effects of exercise on cognitive decline: a preventive approach to Alzheimer disease. Cureus 2020; 12(2):e6958. doi: 10.7759/cureus.6958 [Crossref] [ Google Scholar]