Biomed Res Bull. 1(1):19-29.
doi: 10.34172/biomedrb.2023.06
Systematic Review
Effect of Montelukast on Treatment of Coronavirus Pneumonia (COVID-19): A Systematic Review
Hanieh Salehi-Pourmehr 1  , Sanam Dolati 2, *, Robab Mehdipour 1, Afra Memar 3, Farnaz Ghafourian 3, Avin Shakiba 1, Nasrin Abolhasanpour 1, *
, Sanam Dolati 2, *, Robab Mehdipour 1, Afra Memar 3, Farnaz Ghafourian 3, Avin Shakiba 1, Nasrin Abolhasanpour 1, * 
Author information:
1Research Center for Evidence-Based Medicine, Iranian EBM Centre: A Joanna Briggs Institute (JBI) Center of Excellence, Tabriz University of Medical Sciences, Tabriz, Iran
2Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
The coronavirus disease 2019 (COVID‐19) exhibits the most important global public health emergency. Montelukast (MTL), a prototype cysteinyl leukotriene receptor antagonist, is commonly considered in the therapy of exercise- and aspirin-induced asthma. The purpose of this study was to present a systematic review of the literature on the effectiveness of MTL against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and exuberant immune activation in COVID‐19 disease.
Methods:
PubMed, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials were searched from the database on August 15, 2021and updated on November 19, 2022. Two reviewers independently screened articles, appraised methodological quality, and extracted the data.
Results:
A total of 118 related reports were recognized after eliminating duplicates. Of these, 30 references were screened based on titles and abstracts. After removing unrelated studies, 20 studies were included in the full-text review and evaluated for appropriateness. Finally, eight studies fitting the inclusion criteria for data extraction were selected. One of them was a prospective, randomized, controlled, and single-blinded study, three were open-label randomized or non-randomized controlled clinical trials, two were retrospective studies, and two were case series or comparative studies. A total of 1083 patients infected with COVID-19 infection (999 adults and 84 children) were examined on the effectiveness of MTL on symptom severity as well as hospitalization length. The results of the mentioned studies showed a low risk of clinical deterioration in the MTL group. In addition, the length of hospital stay was low in the treatment group compared to the standard management.
Conclusion:
MTL as a potential adjuvant therapy in COVID-19 may improve lung injury, inflammation, and symptoms. Moreover, the use of MTL could decrease the severity and mortality of COVID-19. Additional well-designed randomized controlled trials are necessary to approve the role of MTL in SARS-CoV-2 prevention or COVID-19 symptoms improvement.
Keywords: Montelukast, SARS coronavirus, Respiratory diseases, Leukotriene receptor antagonist, Inflammation
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
On January 7, 2020, scientists promptly isolated a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1-3 which was the responsible agent for the coronavirus disease 2019 (COVID-19) from confirmed infected pneumonia patients and had more than 95% homology with the bat coronavirus.4,5 SARS-CoV-2-infected pneumonia is commonly displayed as bilateral ground-glass opacities in the lung periphery on chest computerized tomography (CT) scans.6 Acute respiratory distress syndrome (ARDS) is the main cause of death from COVID-19.7 One of the main mechanisms for ARDS is the cytokine storm, a phenomenon of excessive systemic inflammatory reaction in which pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-7, IL-10, IL-12, IL-17, IL-1 receptor antagonist, interferon (IFN)-γ, tumor necrosis factor-alpha (TNF-α), and chemokines (CCL2, CCL3, CCL5, CCL7, CXCL8, CXCL9, CXCL10, and so on) are rapidly produced in large amounts in response to SARS-CoV infection.8,9 Therefore, targeting immune pathways possibly will improve respiratory symptoms, lung function, and the overall prognosis of SARS-CoV-2 infected patients.
Leukotrienes (LTs) are lipid mediators which stimulate airway inflammation, bronchoconstriction, microvascular permeability, and mucus secretion in asthma and chronic obstructive pulmonary disease.10 Additionally, cysteinyl-leukotrienes (CysLTs) are well-known in respiratory medicine. Preclinical and in vitro models recommend that the CysLTs play the main role in airway remodeling.11 It seems that LTs are involved in the pathogenesis of viral pneumonia and ARDS which are common manifestations of COVID-19. The activation of LTs in SARS-CoV-2 infections is associated with a high level of pro-inflammatory cytokines and poor clinical outcomes in patients with severe COVID-19. Thus, the inhibition of the LT pathway could alleviate immune response, pulmonary inflammation, and COVID-19 severity.12 Montelukast (MTL) works as a CysLT receptor antagonist licensed to treat asthma and allergic rhinitis.13 It binds with high affinity and selectivity to the CysLT1 receptor, inhibits the production of reactive oxygen species and leukotriene B4, and also prevents inflammatory cytokine production by blocking the p38 mitogen-activated protein kinases and NF-κB pathways.14 Remarkably, MTL, a safe drug commonly used in asthmatic patients, could be an adjuvant in the treatment of COVID-19 either by acting as an anti-viral drug limiting its replication in the host cells or by improving lung injury and inflammation.15 These promising characteristics of MTL are particularly critical during the COVID-19 pandemic.
We have systematically reviewed the evidence from randomized controlled trials to assess the effectiveness of MTL in the treatment of COVID-19. Understanding the actual benefits of this drug is clinically important for decreasing the rate of clinical deterioration caused by COVID-19.
Materials and Methods
This systematic review was prepared based on the Cochrane Collaboration Handbook and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statements.16 Databases such as PubMed, Cochrane library, Scopus, Web of Sciences, ProQuest, and Google Scholar were searched on August 15, 2021. For this purpose, the following keywords were searched:
(((((“Leukotriene Antagonists”[Mesh]) OR (“Leukotriene Antagonists” [Pharmacological Action])) OR (Leukotriene Antagonist*[Text Word])) OR (“montelukast” [Supplementary Concept])) OR (montelukast [Text Word])) AND (((((((((“COVID-19”[Mesh]) OR (“SARS-CoV-2”[Mesh])) OR (COVID-19[Text Word])) OR (2019-nCoV[Text Word])) OR (2019-CoV[Text Word])) OR (Coronavirus Disease-19[Text Word])) OR (SARS Coronavirus 2[Text Word])) OR (SARS-CoV-2 [Text Word])) OR (SARS-nCoV-2[Text Word])). The search strategy in all databases is attached in Supplementary file 1.
Data Collection and Analysis
Primary Endpoint
The primary endpoint used was the efficacy of MTL in the treatment of COVID-19 symptoms and their severity.
Secondary Endpoint
The secondary endpoint was the length of hospital stay.
Study Screening
Duplicate citations were eliminated before loading all indicated sources into Endnote X9. Then, two impartial reviewers evaluated the titles and abstracts based on the review’s inclusion criteria. Two independent expert reviewers thoroughly examined the entire texts of the chosen eligible studies, and if they did not match the inclusion criteria, the experts would exclude them. Then, the third reviewer or discussion was used to settle any disputes among the reviewers.
Extraction of Data
Two reviewers independently selected the qualified papers and extracted the data from them using the modified standard JBI data extraction tool. Any differences were resolved by consensus between the two reviewers or discussion with the third reviewer.
Evaluation of Methodological Quality
The critical evaluation of eligible research was conducted at the study level by two independent reviewers using standardized critical appraisal instruments from the Joanna Briggs Institute. Further, discussion or conversation with the third reviewer was used to settle any disputes between the reviewers. Studies would be classified as being of good or moderate quality if they had a score of 50% or above on the evaluation questions (found at https://jbi.global/critical-appraisal-tools), as depicted in Table 1.
Table 1.
Methodological quality of the included studies
|
Study*
|
Q1
|
Q2
|
Q3
|
Q4
|
Q5
|
Q6
|
Q7
|
Q8
|
Q9
|
Q10
|
Q11
|
Q12
|
Q13
|
| Mohamed Hussein et al17 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
| Khan et al22 |
Retrospective |
| Kerget et al18 |
Unclear |
Unclear |
Yes |
Yes |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| Soltani et al21 |
Unclear |
Unclear |
Yes |
U |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| Parisi et al20 |
Unclear |
Unclear |
Yes |
No |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
| Norouzi23 |
Yes |
No |
No |
No |
Yes |
Yes |
Yes |
Yes |
Yes |
|
|
|
|
| May and Gallivan19 |
Retrospective |
| Lima-Morales et al24 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Results
The summary of study characteristics included in the systematic review is shown in Tables 2-4. A total of 118 related reports were recognized after eliminating duplicates. Of these, 30 references were screened based on titles and abstracts. After removing unrelated studies, 20 studies were included in the full-text review and evaluated for appropriateness. Finally, eight studies matched the inclusion criteria for data extraction (Figure 1). One of them was a prospective, randomized, controlled, and single-blinded study, three were open-label randomized or non-randomized controlled clinical trials, two were retrospective studies, and two were comparative studies. A total of 1083 patients infected with COVID-19 infection (999 adults and 84 children) were examined on the effectiveness of MTL on symptom severity as well as hospitalization length. Of these, 224 cases received only MTL with different doses of 5 or 20 mg/d for 10-14 days, while 556 patients received it in combination with other medications, including gabapentin (GPT), Leucodif or ivermectin, azithromycin, MTL, and acetylsalicylic acid. The remained cases received standard treatment and were considered the control group. Most of the cases were male in the included studies. The results of the mentioned studies revealed a low risk of clinical deterioration in the MTL group. In addition, the length of hospital stay was low in the treatment group compared to the standard management group.
Table 2.
The Summary of Study Characteristics Included in Systematic Review
|
Study
|
Aim
|
Study Design
|
Population
|
Sample Size
|
Male/Female
|
Mean Age
|
Covid Diagnosis
|
| Mohamed Hussein et al17 |
To assess the effect of 2-week treatment with MTL on both the cough severity and cough-related quality of life among patients with a persistent post-COVID-19 cough. |
Interventional open-label non-randomized controlled pilot trial |
Recovered cases with confirmed COVID-19 with a persistent cough ( > 8 weeks) |
426 |
154/272 (36/64%) |
43 ± 12 (19–73) |
(PCR and/or clinical radiologic laboratory) a positive test for COVID-19 is not a prerequisite for diagnosis |
| Kerget et al18 |
To determine the effectiveness of MTL on COVID-19 patients |
Prospective, randomized, controlled, single-blinded study |
Hospitalized COVID-19 patients in the infectious diseases department |
180 |
Men accounted for 24 patients in the control group, 22 in Group 2, and 30 in Group 3 (P = 0.28). |
54.6 ± 15.3 |
|
| May and Gallivan19 |
To investigate patient outcomes, 53 consecutive COVID-19 test ( + ) cases (ages 3–90) from a well-established, single-center practice in Boston, Massachusetts, between March – November 2020, were treated with levocetirizine and MTL in addition to the existing protocols |
Retrospective single-center practice |
(34 cases were considered mild (64%), 17 moderate (32%), and 2 (4%) severe |
53 |
21/23 |
Males (55) and females (51) |
|
| Parisi et al20 |
i) To evaluate whether the addition of a nutraceutical (Leucodif®) could improve the efficacy of MTL or ICS compared to the single treatment; ii) To verify whether a treatment is more effective than the other |
Multicenter, open-label study |
Children aged 2–6 years diagnosed with recurrent wheezing |
84 |
42/42 |
3.48 ± 1.8 |
|
| Soltani et al21 |
To evaluate the effectiveness of GBT alone and in combination with MTL for improving cough. |
Open-label randomized controlled clinical trial |
Patients hospitalized with moderate to severe COVID-19 who had a cough with a BCSS score of at least 2 based on its cough subscale |
180 |
101 (56.11%) were male |
56.78 (14.48) |
|
| Khan et al22 |
To determine if MTL treatment would reduce the rate of clinical deterioration as measured by the COVID-19 ordinal scale. |
Retrospective analysis |
COVID-19 confirmed hospitalized patients treated with or without MTL |
92 |
15 (50%) in BCSS MTL vs. 38 (61.3%) in the control |
67 (37–93) in MTL vs. 59 (46–75) in control |
|
| Norouzi23 |
To evaluate the therapeutic effects of MTL tablets on COVID-19 patients. |
|
COVID-19 confirmed patients |
20 |
10 males and 10 females |
18 to 82-year-old patients (44.7 ± 17 in men and 41 ± 17.45 years in women) |
PCR and CT-scan |
| Lima-Morales et al24 |
To assess the effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, MTL, and
TNR4 therapy to prevent hospitalization and death among ambulatory COVID-19 cases |
Comparative effectiveness study |
Confirmed SARS-CoV2 cases |
768 |
226 (47.5%) in the TNR4 group vs. 161 (57.8%) in the comparison group |
41.3 (13.5) in TNR4 and 46.2 (14.8) in comparison (18–80 years) |
|
Note. MTL: Montelukast; COVID‐19: Coronavirus disease 2019; PCR: Polymerase chain reaction; ICS: Inhaled corticosteroid; GBT: Gabapentin; BCSS: Breathlessness, cough, and sputum scale; CT: Computerizes tomography; TNR4: Acetylsalicylic acid; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Table 3.
The summary of study characteristics included in systematic review
|
Author
|
Intervention
|
Control
|
Outcomes
|
Other Characteristics
|
Mean Numbers
of Days Needed
for Improvement
|
| Mohamed Hussein et al17 |
32 patients received standard cough therapy + MTL (10 mg/d for 14 days) |
36 patients received only cough therapy |
The duration and severity of the persistent post-COVID-19 cough and a further improvement in the quality of life at day 0 and day 14 of the intervention |
367 (86%) were nonsmokers, only 103 (24.1%) received the influenza vaccine in the preceding year, 162 (38%) were PCR positive, 115 (26.9%) had comorbidities, and 101 (23.7%) required hospital admission. During acute COVID-19, they were treated with antibiotics (86.6%), hydroxychloroquine (26.5%), steroids (44.1%), anticoagulants (48.5%), and vitamins and zinc (100%). The mean duration of recovery was 65 ± 18 (14–120) days. |
5 ± 1.4 in interventional vs. 10 ± 1.5 days in non-interventional group (P < 0.01) |
| Kerget et al18 |
Group 2 (n = 60) received 10 mg/d oral MTL in addition to standard treatment, and group 3 (n = 60) received 20 mg/d oral MTL in addition to standard treatment. |
60 pts with standard treatment in accordance with national COVID-19 diagnosis and treatment guide |
The primary outcome: Progression to ARDS and MAS during follow-up; Secondary outcome: Lung capacity in pulmonary function testing |
The mean ages in the control group and groups 2 and 3 were 54.8 ± 14.8, 54.2 ± 16.5, and 52.8 ± 14.3 years, respectively, with no statistically significant difference between the groups (P= 0.7). Comorbidities were present in 24 patients in the control group, 28 patients in group 2, and 26 patients in group 3 (P = 0.16). Hypertension was observed in 20 patients in the control group, 20 patients in group 2, and 14 patients in group 3 (P= 0.15), while diabetes was present in 11 patients in the control group, 12 patients in group 2, and 11 patients in group 3 (P = 0.45). Two patients in group 3 had coronary artery disease, one patient in each group had asthma, and one patient in group 2 had a psychiatric disease. |
|
| May and Gallivan19 |
|
|
|
Several patients presented with significant comorbidities (obesity: n = 22, 41%; diabetes: n = 10, 19%; and hypertension: n = 24, 45%). During the illness 66% had a fever (n = 35; > 100.4 ◦F, 38°C), 50% had a headache (n = 25/50), and 29% had a loss of the sense of smell/taste (n = 15/52). |
|
| Parisi et al20 |
ii) 22 pts MTL; iii) 24 pts MTL + Leucodif; iv) 20 pts ICS + leucodif. |
18 pts ICS treatment |
At baseline and after one, two, and three months of treatment using the TRACK score for both the caregiver and the physician |
|
|
| Soltani et al21 |
G1: 76 pts GBT/5 days and G2: 51 pts GBT/MTL/5days |
53 pts dextromethorphan (DXM)/5days |
The severity of the cough was evaluated using the BCSS scale and VAS |
|
|
| Khan et al22 |
30 who received MTL at the discretion of the treating physician |
62 patients who did not receive MTL. |
Any increase in the ordinal scale value from day 1 to day 3 of the hospital stay (clinical deterioration) |
|
|
| Norouzi23 |
MTL (10 mg) tablet for 10 days. |
|
|
The frequency of respiratory distress, cough, abdominal cramps/diarrhea, fever, and odor disorder clinical signs amongst the examined patients were 85%, 90%, 20%, 70%, and 65%, respectively |
|
| Lima-Morales et al24 |
|
481 cases received the TNR4 therapy |
287 received another treatment (comparison group). |
Recovery within 14 days after the onset of symptoms: OR: 3.4 (2.38–4.96; P = 0.000) compared to the comparison group |
|
Note. MTL: Montelukast; COVID‐19: Coronavirus disease 2019; PCR: Polymerase chain reaction; Polymerase Chain Reaction; ARDS: Acute respiratory distress syndrome; MAS: Macrophage activation syndrome; ICS: Inhaled corticosteroid; TRACK: Test for respiratory and asthma control in kids; BCSS: Breathlessness, cough, and sputum scale; VAS: Visual analog scale; TNR4: Acetylsalicylic acid; OR: Odds ratio.
Table 4.
The summary of study characteristics included in systematic review
|
Study
|
The Length of Hospitalization in Days
|
Clinical Deterioration (Any Escalation in Ordinal Scale Value Day 1 to Day 3)
|
Hospitalization Rate
|
Death
|
Outcome
|
|
MTL Therapy
|
Standard Therapy
|
MTL Therapy
|
| Mohamed Hussein et al17 |
7 (4–10.5) |
8 (6–12) |
|
|
|
There was a considerable improvement in the number of paroxysms/days in the interventional group (P < 0.01), cough severity VAS (P < 0.001), cough severity index (P < 0.01), and cough quality of life (P < 0.01). |
| Kerget et al18 |
9.4 ± 2.1 in 10 mg, 9.3 ± 3.6 in 20 mg |
11 ± 5.3 |
MTL is known to have both anti-inflammatory and bronchodilatory activity and is reported to be safe even at high doses. Its use as an adjunct therapy may prevent the development of MAS in COVID-19 patients due to its anti-inflammatory effects, while its bronchodilator effect at high doses may help minimize respiratory failure in these patients and reduce their length of hospital stay. On Day 5 of the treatment, only Group 3 showed significant improvement in FEV1, FVC, and PEF25–75 values compared with admission (P = 0.001 for all). |
|
|
|
| May and Gallivan19 |
Fifty-one of 53 patients received a clinical cure on therapy with the restoration of their overall status to a pre-infection baseline within two weeks. No patient progressed to intubation or death. |
|
|
|
|
|
| Parisi et al20 |
All four treatments resulted in a significant reduction in symptoms with no differences among the various groups |
|
|
|
|
|
| Soltani et al21 |
10 (7) in GPT, 8 (3) in GPT/MTL, respectively |
9 (5) and |
GPT, both alone and in combination with MTL, improved cough frequency and severity in hospitalized patients with COVID-19, with the combination being more efficacious. This regimen may be useful in patients who cannot tolerate opioids. BCSS [mean (SD)]: Change from baseline: 1.47 (0.81) in GPT, 1.96 (0.69) in GPT/MTL, and 2.89 (0.32) in DXM; VAS [mean (SD)] change from baseline: 1.43 (1.13) in GPT, 1.80 (1.11) in GPT/MTL, and 2.37 (0.82) in DXM |
|
|
|
| Khan et al22 |
|
3 (10) |
20 (32.2) |
|
Patients receiving MTL experienced significantly fewer events of clinical deterioration compared with patients not receiving MTL (10% vs. 32%, P = 0.022). |
|
| Norouzi23 |
All patients who received 10 days of oral administration of MTL tablets (10 mg) recovered from the COVID-19 disease. Additionally, all of the clinical signs of COVID-19 patients, including respiratory distress, cough, and odor disorder gradually disappeared. Our findings revealed that the widespread oral administration of MTL tablets (10 mg) is a potential treatment for COVID-19 disease. |
|
|
|
|
|
| Lima-Morales et al24 |
Hospitalization within 14 days after the onset of symptoms: OR: 0.25 (0.16–0.38; P = 0.000) compared to the comparison group. |
|
|
9.1% (n = 44/481) in the TNR4 vs. 31.0% (n = 89/287) in the comparison group |
3.1% (n = 15/481) in the TNR4 vs. 18.1% (n = 52/287) in the comparison group within 14 days after the onset of symptoms: OR: 0.19 (0.10–0.36; P = 0.000) |
Nearly 85% of cases who received the TNR4 recovered within 14 days compared to 59% in the
comparison group. The likelihood of recovery within 14 days was 3.4 times greater among the TNR4
group than in the comparison group. Patients treated with TNR4 had a 75% and 81% lower risk of being hospitalized or death, respectively, than the comparison group. |
Note. MTL: Montelukast; VAS: Visual analog scale; MAS: Macrophage activation syndrome; COVID-19: Coronavirus disease 2019; FEV1: Forced expiratory volume during the first second, FVC: Forced vital capacity; PEF25: Peak expiratory flow 25–75; GPT: Gabapentin; SD: Standard deviation; DXM: Dextromethorphan; OR: Odds ratio; TNR4: Acetylsalicylic acid.
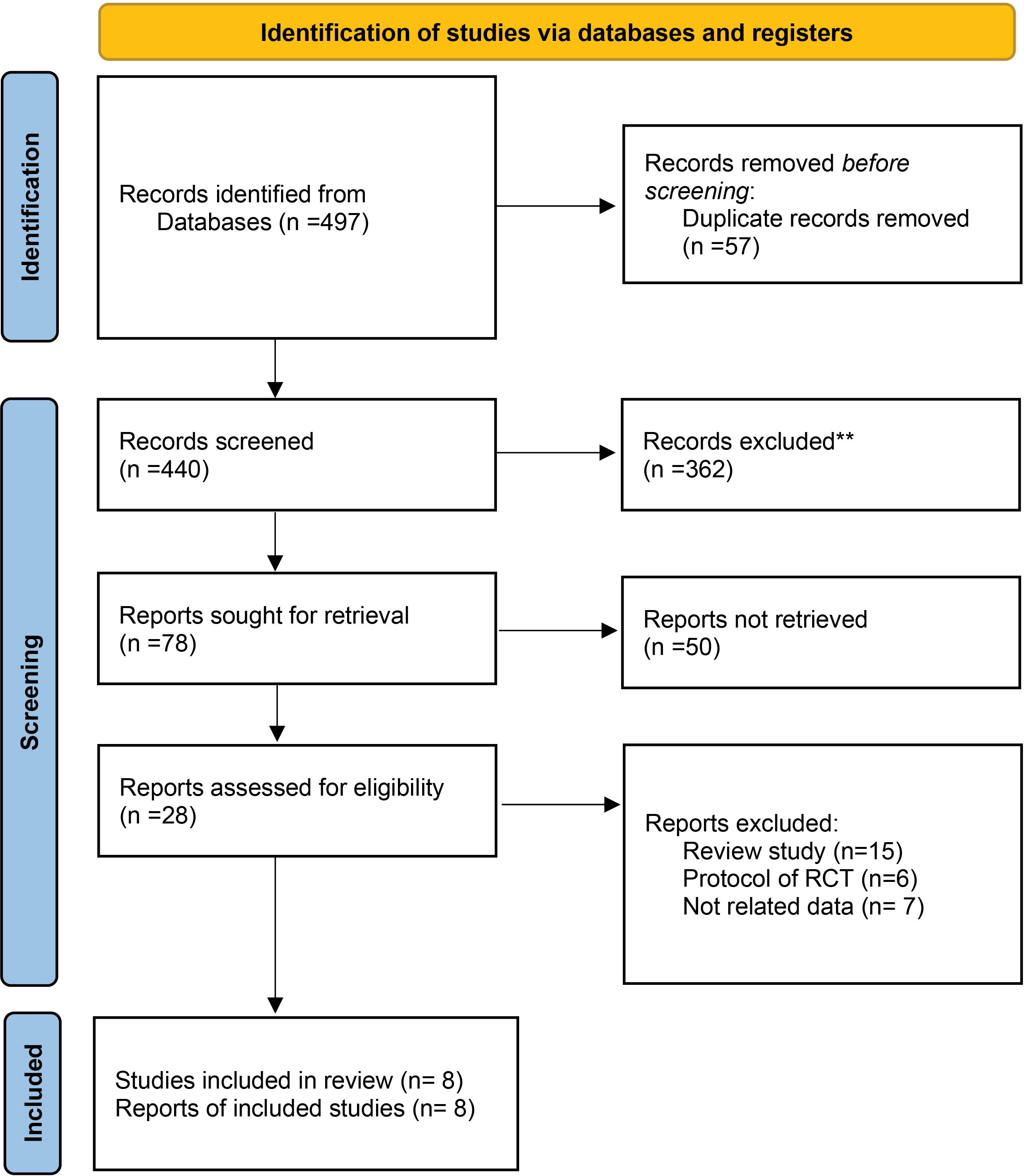
Figure 1.
The flowchart of searching and selecting the final studies (16)
.
The flowchart of searching and selecting the final studies (16)
Description of Studies
In the open-label non-randomized controlled pilot study on 68 cases with post-COVID-19 persistent cough ( > 8 weeks), the intervention group included 32 patients who received standard cough therapy and MTL (10 mg/d) for 14 days, and 36 patients in the control group received only cough sedatives.17 In the studied groups, the CT chest and polymerase chain reaction (PCR) findings were unremarkable. The ratio of male/female was 36/64%, and their mean age was 43 ± 12 (19–73) years old. During acute COVID-19, patients received treatment by antibiotics (86.6%), hydroxychloroquine (26.5%), steroids (44.1%), anticoagulants (48.5%), and vitamins and zinc (100%). The mean duration of recovery in patients was 65 ± 18 (14–120) days.
Following treatment with 10 mg MTL for 14 days, considerable improvement was observed in the number of paroxysms/days in the interventional group (P< 0.01), cough severity index (P< 0.01), cough quality of life (P< 0.01), and visual analog scale (VAS) for cough severity (P< 0.001). Furthermore, the mean number of days for complete improvement was 5 ± 1.4 in the interventional group, while it was 10 ± 1.5 days in the noninterventional group (P< 0.01). Moreover, side effects were observed in 18.7% of cases (17).
In the study by Kerget et al,18 180 participants were monitored and divided into three groups. Group 1 received standard treatment, and groups 2 and 3 underwent standard treatment and 10 mg/d oral or 20 mg/d MTL. Laboratory parameters on day 5 of treatment indicated a significant reduction in D-dimer, lactate dehydrogenase, fibrinogen, procalcitonin, and C-reactive protein levels in groups 2 and 3 compared to group 1. In addition, a comparison between groups 2 and 3 showed a significantly lower rate of fibrinogen level in group 3 (P= 0.02). Logistic regression analysis also reported a significant positive change in fibrinogen and peak expiratory flow 25–75 (PEF25–75) levels only in group 3 (P= 0.05, 0.001). However, on day 5 of treatment, only group 3 exhibited significant improvement in forced expiratory volume during the first second, forced vital capacity, and PEF25–75 values compared with admission (P= 0.001) for all. In terms of COVID-19 severity, a significantly lower rate of progression to macrophage activation syndrome and respiratory failure was observed in groups 2 and 3 compared to group 1 (P= 0.001). Moreover, mortality was observed only in group 1 with four patients (6.7%), and the length of hospital stay was significantly lower in groups 2 and 3 than in group 1 (P= 0.03).
May and Gallivan19 investigated the effect of combination therapy with levocetirizine and MTL on treating COVID-19. For this purpose, 53 cases with positive COVID-19 tests (ages 3–90) from a single-center practice in Boston, Massachusetts, were enrolled in the study during March-November 2020. They received combination therapy in addition to the existing protocols. The patients include 32 females (mean age 51) and 21 males (mean age 55). Thirty-four patients (64%) were considered mild, 17 (32%) moderate, and 2 (4%) severe. Remdesivir was also administered to the two severe hospital cases. No patient underwent intubation, and the number of deaths was zero. Further, co-existing morbidities (e.g., obesity, hypertension, and diabetes) were observed in most allergy and asthma patients; however, nobody recovered well from the virus. In addition, early therapy, mainly in younger cases, increased the clinical response with the highest resolution of both fever and headache during the first 48 hours after the initiation of the treatment. The overall analysis demonstrated an improvement in the patients who received combination therapy with levocetirizine and MTL compared to patients who only received the existing protocols or were left untreated. In most patients that received combination therapy, a resolution was observed within seven days, while it lasted for 10-14 days in other untreated patients.
Parisi et al20 performed a multicentral randomized controlled trial on 84 children with a mean age of 3.48 ± 1.8 years. They divided patients into four groups: 18 patients received inhaled corticosteroid (ICS) (beclomethasone dipropionate 100 μg x 2 given through a spacer device) therapy, 24 patients received MTL (4 or 5 mg/d according to child’s age), 22 patients received ICS + Leucodif (one capsule/day for 14 days/month for three months), and 20 patients received MTL + Leucodif. All patient groups received therapeutic regimens for three months. A clinical check was expected with the administration of the test for respiratory and asthma control in kids (TRACK) for children at four different times. The results indicated that all four treatments reduced the symptoms significantly with no differences among the groups. They concluded that MTL therapy has an equal effect on ICS therapy.
An open-label randomized controlled clinical trial was conducted by Soltani et al21 at Al-Zahra hospital of Isfahan, Iran, during April-May 2020. Of 180 patients, 76 patients were in the GPT group (300-mg oral capsules of GPT every 8 hours), 51 in MTL/GPT (300-mg oral capsules of GPT every 8 hours and 10-mg oral tablets of MTL every evening) for 5 days, and 53 in dextromethorphan (DXM) group (30 mg oral syrup of DXM every 8 hours) for 5 days. Regarding breathlessness, cough, and sputum scale and VAS scores in all groups, a significant reduction was observed in baseline values (P< 0.0001), while the change rate was significantly higher in the DXM group. The reduction level in breathlessness, cough, and sputum scale in the GPT/MTL group was significantly higher than that in the GPT group, but there was no significant difference in the VAS score of these two groups. Moreover, the GPT/MTL group showed shorter hospitalization duration, and a significant difference was observed between the GPT and GPT/MTL groups (P< 0.0001).
Khan et al22 performed a retrospective study at Robert Wood Johnson University Hospital in New Brunswick. From late March to early April 2020, upon admission, patients received MTL at the authorization of the treatment provider. Two patient groups were identified: 30 patients who received MTL and 62 patients as the control group that did not receive MTL. Three days have passed since all patients’ hospitalization. MTL treatment was started on day 1 of hospitalization with the current standard dose of 10 mg orally once a day with no previous therapy with MTL. The control patients were selected from confirmed COVID-19 patients who survived up to at least the fourth day of admission. Patients who took an ordinal scale score of three or higher indicated the need for hospitalization enrolled in the study. A more significant proportion of the MTL-received patients had asthma (36.7% vs. 6.5%, P= 0.0005) and received a lower rate of azithromycin compared to the control group (13% vs. 40%, P= 0.009). Lower baseline lactate dehydrogenase was observed in the MTL group (median 344.5 vs. 439, P = 0.04). Patients who received MTL experienced significantly less clinical deterioration (10% vs. 32.2%, P = 0.022), including any escalation in the ordinal scale value from day 1 to day 3. Further, based on univariate logistic regression, the risk of clinical deterioration was low in the MTL group (odds ratio [OR] = 0.23, P = 0.029, confidence interval = 0.063–0.86). Following multivariable logistic regression and accounting for age ≥ 60 (binary), a slight association was observed between receiving MTL and a lower risk of clinical deterioration (OR = 0.28, P = 0.058, confidence interval = 0.072–1.04). In addition, sensitivity analysis indicated fewer clinical deterioration events among patients without asthma that received MTL (11% vs. 33%, P = 0.077). Furthermore, a reduction was found in the clinical deterioration events by sensitivity analysis among patients who did not receive azithromycin (8% vs. 32%, P = 0.030). Overall, this study indicated that treatment by MTL in hospitalized COVID-19 patients leads to fewer events of clinical deterioration, as demonstrated by the COVID-19 ordinal scale.
Another study was performed by Norouzi23 to evaluate the MTL treatment in patients diagnosed with the COVID-19 virus. For this purpose, a total of 20 male and female patients (male = 10 and female = 10) aged 18-82 years were enrolled in the study. In addition to the clinical signs of diseases, the patients were confirmed by both positive outcomes of real-time PCR and CT scan. Patients with progressive and autoimmune diseases who used other SARS-CoV-2 therapeutic options and died during the study were excluded from the research. Following the confirmation of patients by CT scan and reverse transcription real-time PCR, the oral administration of MTL tablets (10 mg) was applied for about 10 days. Patients received two MTL tablets on the first day of research and continued with one tablet from days 2 to 10 of the study. Furthermore, all patients received routine care for COVID-19.
CT-scan images showed the multi-lobar and bilateral ground-glass opacities in both lungs. However, all lobes were affected, but the highest frequency was observed in mid to lower lungs and peripheral subpleural distribution. The mean age of males and females was 44.7 ± 17 and 41 ± 17.45 years, respectively. Respiratory distress, fever, cough, abdominal cramps/diarrhea, and odor disorders distribution among patients were 85%, 70%, 90%, 20%, and 65%, respectively. Any case with progressive and autoimmune diseases or deaths was reported in the study. The findings of this research revealed a gradual improvement in clinical signs of COVID-19 in patients such as respiratory distress, cough, and odor disorders. Following the MTL administration, the first clinical signs that disappeared were cough and respiratory distress. Nevertheless, signs such as cramps and diarrhea continued for about a few days after the study experiment. Patients’ odor appeared about one to two days after the end of the study period. Moreover, no detectable side effects were reported after ten days of oral administration of MTL tablets.
Lima-Morales et al24 conducted a comparative study on patients with laboratory-confirmed SARS-CoV-2 virus at the Ministry of Health of Tlaxcala. In the time interval between May 11 to September 9, 2020, from 6798 laboratory-confirmed COVID-19 cases, 1147 eligible ambulatory patients with mild or moderate symptoms of COVID-19 infection were invited to participate in the study. After excluding the patients under 18 or over 80 years (n = 44), those who refused to participate (n = 251) and those who took treatment on the same day or the previous day were hospitalized or died (n = 84). Finally, 768 COVID-19 patients were enrolled in the study, 481 were accepted to receive the acetylsalicylic acid (TNR4) therapy, and 287 patients received another treatment (comparison group). All participants completed a questionnaire, and follow-ups were made by phone calls or visits at home during 14 days of clinical evaluation. The TNR4 multidrug therapy contains four drugs used for patients with mild or moderate symptoms, which are all administrated orally, including MTL (60 mg on the first day and continued by 10 mg between days 2–21), ivermectin (12 mg single dose), azithromycin (500 mg for four days), and acetylsalicylic acid (100 mg for 30 days).
Participants in the TNR4 group were 10.3% less likely to be male, exhibited a 7.1% lower prevalence of different comorbidity, were 4.9 years younger than the comparison group, and were 8.4% more likely to be health workers (P≤ 0.050 in all cases). Moreover, a significantly high percentage of recovered patients by day 14 (84.4%) was observed in the TNR4 group compared to the comparison group (58.9%). Furthermore, in the TNR4 group a higher percentage of fully recovered patients after 14 days was reported in comparison to other treatment strategies in the following sub-groups: older participants with the age of 49–80 years (TNR4 76.4% vs. comparison group 45.4%), patients with various comorbidities (TNR4 74.6% vs. comparison group 41.2%), health workers (TNR4 86.5% vs. comparison group 60.5%), and male gender (TNR4 85.4% vs. comparison group 57.8%) with P≤ 0.001 in all groups. Hospitalization among patients who received TNR4 therapy revealed a significantly lower percentage than the comparison group (in all sub-groups, P≤ 0.010). Further, the rate of dead participants in the TNR4 group was markedly lower than that in the comparison group for all sub-groups (P≤ 0.027 in all groups).
Regarding the study sub-groups, the findings showed the same result: a 3.5 times higher likelihood of recovery in participants with different comorbidities, a 3.0- and 4.0-fold greater possibility of complete recovery in the young and old age group, and a 3.9 and 1.9 higher probability of recovery in both males and health workers (P≤ 0.005 in all groups except for the health workers’ group). Among the study participants, hospitalization risk in the TNR4-treated patients was 75% lower than that in the comparison group (OR: 0.25). Additionally, lower hospitalization risk was observed in patients with comorbidities (76%, OR: 0.24), younger patients (77%, OR: 0.23), older patients (68%, OR: 0.32), males, and health workers.
Patients with comorbidities exhibited a 76% lower risk of hospitalization (OR: 0.24), and a lower risk was observed in younger (77%, OR: 0.23) and older (68%, OR: 0.32) participants as well as in males (76%, OR: 0.24) and health workers (66%, OR: 0.34) compared to the comparison group (P≤ 0.000 in all groups, with the exception in the health workers group).
Moreover, participants who received TNR4 reported an 81% lower risk of death among the whole study population (OR: 0.19). In addition, a lower risk of dying was reported in patients with comorbidities (78%, OR: 0.22), middle-aged patients (94%, OR: 0.06), older patients (78%, OR: 0.22), and males (82%, OR: 0.18) compared to the comparison group (P≤ 0.008 in all groups).
Of 481 COVID-19 patients treated with TNR4, 90.3% received all four drugs, 8.3% received three drugs, and 1.4% received only one medication. The 354 participants who took four TNR4 medications showed some common undesirable side effects, but most patients did not experience side effects from any of the four drugs. The most frequently reported adverse effects in the patients who received combination therapy were gastrointestinal disorders such as vomiting, abdominal pain, diarrhea, nausea, and constipation (5.9%), headache symptoms and dizziness (3.1%), fatigue, confusion, and asthenia (1.4%), and urticarial and itchy feeling (0.9%).
Most patients treated with TNR4 medications did not undergo any other treatments (89.8%). In comparison, 2.71% of this population used non-steroidal anti-inflammatory drugs (NSAIDs), 1.90% took therapy to treat cold and flu signs, 0.9% received antibiotics, and 0.74% used antivirals. Of COVID-19 patients with no TNR4 treatment, 19% did not receive any medications, 61.4% received NSAIDs, 14.4% used combined antibiotics with NSAIDs or corticosteroids, and 5.2% received antiviral drugs accompanied by NSAIDs or different corticosteroids. The findings of this study indicated that TNR4 multidrug therapy can be effective in the recovery of patients and decrease the risk of severe disease amongst ambulatory cases of COVID-19. Supplementary File 1 represents the other included study outcomes.
Discussion
The COVID-19 pandemic is an outstanding devastating disease, and there has not been any specific therapy approved to control the clinical symptoms of the disease up to now. LTs may be involved in the pathology of the aberrant immune response against SARS-CoV-2. The inhibition of LT pathways may be a probable target in the treatment of COVID-19.25 This study systematically reviewed the probable therapeutic potential benefits of using MTL to manage COVID-19 pathophysiology. MTL has revealed anti-inflammatory effects, antioxidant properties, decreased cytokine production, and improved lung complications. Indeed, MTL with these properties may limit the progression of the COVID-19 infection.
MTL as a CysLTR antagonist has had clinical applications for asthma for more than two decades.26,27 MTL has been revealed to decrease both cytokine release and lung inflammation in preclinical models of viral influenza and ARDS.28 In a preclinical study by Cardani et al,29 leukotriene receptor antagonists inhibited the development of fatal influenza pneumonia in mice with influenza infection. A retrospective observational study indicated a statistically substantial decrease in confirmed COVID-19 patients amongst the elderly asthmatic patients treated with MTL.30 Moreover, it has been hypothesized that MTL could limit the progression of the disease for COVID-19-positive cases, particularly in patients with central obesity and metabolic syndrome.30 Based on the results of a study by Khan et al, no differences were observed in inflammatory laboratory values such as C-reactive protein, D-dimer, and ferritin between MTL versus non-MTL patients. Moreover, no difference was found in the length of hospitalization between the two groups.22 TNR4 is a multidrug therapy (ivermectin, azithromycin, MTL, and ASA) for COVID-19 cases.24 TNR4 significantly increased the likelihood of full recovery 3.4 times within 14 days after the onset of symptoms and decreased the risk of hospitalization by 75% or death by 81% among ambulatory cases of COVID-19.24 Ten properties of MTL will be possibly found as many synergistic and potentiating therapeutic prospects in COVID-19, including antiviral properties, the inhibition of endotheliitis, the inhibition of neurological disorders related to SARS-CoV-2, improvement of atherogenic vascular inflammation, limitation of the ischemia/reperfusion phenomenon, amelioration of respiratory symptoms, limitation of the cytokine storm, alleviation of ARDS, antioxidant properties, and anti-fibrosis effects.31 This review study has some limitations. The findings of this study may not be generalizable since heterogeneous studies with different sample sizes, dosages, or duration of treatment with different control arms were included.
Conclusion
MTL could have a clinical likelihood to control lung pathology during COVID-19. MTL administration in hospitalized patients with confirmed COVID-19 infection revealed anti-inflammatory effects, suppressed oxidative stress, decreased cytokine production, and limited the progression of the disease on COVID-19 infection. Hence, the use of MTL as a therapeutic approach could decrease the severity and mortality of COVID-19. Our study suggests that additional research is needed regarding the role of MTL in SARS-CoV-2 prevention or COVID-19 amelioration. We hope that these studies possibly will support the development of novel therapeutic opportunities for the treatment of COVID-19.
Acknowledgments
The authors would like to thank the Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran and the Evidence-based Medical Research Center, Tabriz University of Medical Sciences, Tabriz, Iran for supporting this study.
Competing Interests
There is no conflict of interests.
Ethical Approval
Not applicable.
Funding
Not applicable.
Supplementary Files
Supplementary file 1 depicts search strategy methods in PubMed and Cochrane databases.
(pdf)
References
- Mostafaei A, Hajebrahimi S, Sadeghi-Ghyassi F, Mostafaei H, Abolhasanpour N, Nasseri A. Can wearing a face mask protect from COVID-19? A systematic review. Iran J Med Microbiol 2020; 14(2):101-7. doi: 10.30699/ijmm.14.2.101 [Crossref] [ Google Scholar]
- Mostafaei A, Ghojazadeh M, Hajebrahimi S, Abolhasanpour N, Salehi-Pourmehr H. Clinical presentation of Iranian patients affected with COVID-19: a thousand faces disease. Iran J Allergy Asthma Immunol 2021; 20(2):140-6. [ Google Scholar]
- Shahsavarinia K, Faridaalaee G, Soleimanpour H, Sadeghi-Ghyassi F, Atashgahi S, Milanchian N. Cerebral venous thrombosis (CVT) Following COVID-19 vaccination: an umbrella review of systematic reviews. Iran J Med Microbiol 2023; 17(1):7-22. [ Google Scholar]
- Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J Gen Intern Med 2020; 35(5):1545-9. doi: 10.1007/s11606-020-05762-w [Crossref] [ Google Scholar]
- Wu P, Hao X, Lau EHY, Wong JY, Leung KSM, Wu JT. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill 2020; 25(3):2000044. doi: 10.2807/1560-7917.es.2020.25.3.2000044 [Crossref] [ Google Scholar]
- Kim H. Outbreak of novel coronavirus (COVID-19): what is the role of radiologists?. Eur Radiol 2020; 30(6):3266-7. doi: 10.1007/s00330-020-06748-2 [Crossref] [ Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8(4):420-2. doi: 10.1016/s2213-2600(20)30076-x [Crossref] [ Google Scholar]
- Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol 2020; 20(5):277. doi: 10.1038/s41577-020-0305-6 [Crossref] [ Google Scholar]
- Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 2020; 251(3):228-48. doi: 10.1002/path.5471 [Crossref] [ Google Scholar]
- Hallstrand TS, Henderson WR Jr. An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol 2010; 10(1):60-6. doi: 10.1097/ACI.0b013e32833489c3 [Crossref] [ Google Scholar]
- Okunishi K, Peters-Golden M. Leukotrienes and airway inflammation. Biochim Biophys Acta 2011; 1810(11):1096-102. doi: 10.1016/j.bbagen.2011.02.005 [Crossref] [ Google Scholar]
- Al-Kuraishy HM, Al-Gareeb AI, Almulaiky YQ, Cruz-Martins N, El-Saber Batiha G. Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of COVID-19: the enigmatic entity. Eur J Pharmacol 2021; 904:174196. doi: 10.1016/j.ejphar.2021.174196 [Crossref] [ Google Scholar]
- Chen Y, Li Y, Wang X, Zou P. Montelukast, an anti-asthmatic drug, inhibits Zika virus infection by disrupting viral integrity. Front Microbiol 2019; 10:3079. doi: 10.3389/fmicb.2019.03079 [Crossref] [ Google Scholar]
- Lin YC, Huang MY, Lee MS, Hsieh CC, Kuo HF, Kuo CH. Effects of montelukast on M2-related cytokine and chemokine in M2 macrophages. J Microbiol Immunol Infect 2018; 51(1):18-26. doi: 10.1016/j.jmii.2016.04.005 [Crossref] [ Google Scholar]
- Tavares M, Farraia M, Silva S, Ribeiro AM, Severo M, Paciência I. Impact of montelukast as add on treatment to the novel coronavirus pneumonia (COVID-19): protocol for an investigator-initiated open labeled randomized controlled pragmatic trial. Porto Biomed J 2021; 6(2):e134. doi: 10.1097/j.pbj.0000000000000134 [Crossref] [ Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021; 88:105906. doi: 10.1016/j.ijsu.2021.105906 [Crossref] [ Google Scholar]
- Mohamed Hussein AA, Ibrahim ME, Makhlouf HA, Makhlouf NA, Abd-Elaal HK, Kholief K. Value of montelukast as a potential treatment of post-COVID-19 persistent cough: a non-randomized controlled pilot study. Egypt J Bronchol 2022; 16(1):1-5. [ Google Scholar]
- Kerget B, Kerget F, Aydın M, Karaşahin Ö. Effect of montelukast therapy on clinical course, pulmonary function, and mortality in patients with COVID-19. J Med Virol 2022; 94(5):1950-8. doi: 10.1002/jmv.27552 [Crossref] [ Google Scholar]
- May BC, Gallivan KH. Levocetirizine and montelukast in the COVID-19 treatment paradigm. Int Immunopharmacol 2022; 103:108412. doi: 10.1016/j.intimp.2021.108412 [Crossref] [ Google Scholar]
- Parisi GF, Manti S, Papale M, Giallongo A, Indolfi C, Miraglia Del Giudice M. Addition of a nutraceutical to montelukast or inhaled steroid in the treatment of wheezing during COVID-19 pandemic: a multicenter, open-label, randomized controlled trial. Acta Biomed 2022; 93(2):e2022156. doi: 10.23750/abm.v93i2.11958 [Crossref] [ Google Scholar]
- Soltani R, Nasirharandi S, Khorvash F, Nasirian M, Dolatshahi K, Hakamifard A. The effectiveness of gabapentin and gabapentin/montelukast combination compared with dextromethorphan in the improvement of COVID-19- related cough: a randomized, controlled clinical trial. Clin Respir J 2022; 16(9):604-10. doi: 10.1111/crj.13529 [Crossref] [ Google Scholar]
- Khan AR, Misdary C, Yegya-Raman N, Kim S, Narayanan N, Siddiqui S. Montelukast in hospitalized patients diagnosed with COVID-19. J Asthma 2022; 59(4):780-6. doi: 10.1080/02770903.2021.1881967 [Crossref] [ Google Scholar]
- Norouzi A. Treatment of SARS-CoV-2 (COVID-19) cases by the oral administration of montelukast tablets. Rev Bionatura 2020; 5(4):1304-8. doi: 10.21931/rb/2020.05.04.5 [Crossref] [ Google Scholar]
- Lima-Morales R, Méndez-Hernández P, Flores YN, Osorno-Romero P, Sancho-Hernández CR, Cuecuecha-Rugerio E. Effectiveness of a multidrug therapy consisting of Ivermectin, Azithromycin, Montelukast, and Acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico. Int J Infect Dis 2021; 105:598-605. doi: 10.1016/j.ijid.2021.02.014 [Crossref] [ Google Scholar]
- Funk CD, Ardakani A. A novel strategy to mitigate the hyperinflammatory response to COVID-19 by targeting leukotrienes. Front Pharmacol 2020; 11:1214. doi: 10.3389/fphar.2020.01214 [Crossref] [ Google Scholar]
- Aigner L, Pietrantonio F, Bessa de Sousa DM, Michael J, Schuster D, Reitsamer HA. The leukotriene receptor antagonist montelukast as a potential COVID-19 therapeutic. Front Mol Biosci 2020; 7:610132. doi: 10.3389/fmolb.2020.610132 [Crossref] [ Google Scholar]
- Bozek A, Winterstein J. Montelukast’s ability to fight COVID-19 infection. J Asthma 2021; 58(10):1348-9. doi: 10.1080/02770903.2020.1786112 [Crossref] [ Google Scholar]
- Cingi C, Muluk NB, Ipci K, Şahin E. Antileukotrienes in upper airway inflammatory diseases. Curr Allergy Asthma Rep 2015; 15(11):64. doi: 10.1007/s11882-015-0564-7 [Crossref] [ Google Scholar]
- Cardani A, Boulton A, Kim TS, Braciale TJ. Alveolar macrophages prevent lethal influenza pneumonia by inhibiting infection of type-1 alveolar epithelial cells. PLoS Pathog 2017; 13(1):e1006140. doi: 10.1371/journal.ppat.1006140 [Crossref] [ Google Scholar]
- Almerie MQ, Kerrigan DD. The association between obesity and poor outcome after COVID-19 indicates a potential therapeutic role for montelukast. Med Hypotheses 2020; 143:109883. doi: 10.1016/j.mehy.2020.109883 [Crossref] [ Google Scholar]
- Barré J, Sabatier JM, Annweiler C. Montelukast drug may improve COVID-19 prognosis: a review of evidence. Front Pharmacol 2020; 11:1344. doi: 10.3389/fphar.2020.01344 [Crossref] [ Google Scholar]