Biomed Res Bull. 2(4):185-187.
doi: 10.34172/biomedrb.2024.25
Study Protocol
A Video-Documented Mouse Model of Internal Capsule Stroke Induced by Photothrombotic Method Using Fiber Optic Technology: A Novel Approach
Mahsa Hasanzadeh-Moghadam 1, 2  , Faraz Norouzi-Bonab 3, Kimia Zabihi 3, Kimia Motlagh Asghari 1, Seyed Zanyar Athari 1, 4, *
, Faraz Norouzi-Bonab 3, Kimia Zabihi 3, Kimia Motlagh Asghari 1, Seyed Zanyar Athari 1, 4, * 
Author information:
1Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Anatomical Sciences, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
3Pharmacy, Eastern Mediterranean University, Famagusta, TRNC via Mersin 10, Turkey
4Department of Medical Physiology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
The internal capsule is a critical structure that is highly susceptible to ischemic injury, yet traditional stroke models often fail to isolate and precisely target this region. Laser-based methods offer a novel approach for inducing controlled, localized ischemia with high spatial accuracy. This study aimed to develop a precise mouse model of internal capsule stroke induced by the photothrombotic method using fiber optic technology, providing a reproducible platform for studying ischemic injury and recovery mechanisms in this vital brain region. To this end, an adult male BALB/c mouse was anesthetized, and 150 μg Rose Bengal/g dissolved in normal saline was intraperitoneally injected for photothrombotic ischemia induction. The optic fiber was positioned into the brain’s internal capsule using a stereotaxic frame. The laser was activated for 10 minutes to induce ischemia, and stroke was validated three days post-procedure using 2,3,5-triphenyl-tetrazolium chloride staining to confirm lesion location and extent. This novel photothrombotic stroke model provides a powerful and reliable tool for investigating ischemic injury and recovery in the internal capsule. The precision and reproducibility of this method make it a significant advancement over traditional stroke models, with potential applications in understanding stroke pathophysiology and evaluating therapeutic interventions.
Keywords: Ischemic stroke, Internal capsule, Photothrombotic ischemia, Mouse
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was self-funded by the authors and received no external financial support from any funding organization.
Introduction
Stroke, an acute neurological event caused by the interruption of blood flow to the brain, is the third leading cause of mortality and the fourth leading cause of disability-adjusted life-year worldwide.1,2 It has devastating effects on brain function and is a major contributor to long-term disability.3,4 The internal capsule, a critical area housing sensory and motor networks, is a significant risk site in brain ischemia. Injury to this area can significantly hinder motor coordination, sensory processing, and other essential brain activities. This vulnerability makes the internal capsule a focal point for stroke research, as understanding its response to ischemic damage can offer insights into stroke pathophysiology and recovery.5-8
The investigation of stroke predominantly relies on various animal models, such as the middle cerebral artery occlusion and mechanical injury techniques.9,10 Although these methodologies have yielded significant discoveries, they include inherent limits. They often struggle with precision in targeting specific brain regions, leading to variability in the size and location of induced infarcts.9-11 These challenges hinder reproducibility and limit the scope of targeted investigations into stroke mechanisms and recovery pathways. The necessity for enhanced models facilitating accurate, localized brain damage assessment has become more evident. By simulating the intricate and regional consequences of stroke, such models would enable researchers to gain a deeper understanding of its effects and possible treatment strategies.
Recent advancements in laser-based technologies have provided new tools for investigating brain function and pathology. The photothrombotic method using fiber optics has emerged as a powerful technique in neuroscience, offering unparalleled spatial and temporal precision.12,13 This method enables researchers to target specific brain regions with minimal collateral damage, making them ideal for studies that require controlled, reproducible conditions.14 Photothrombotic models in stroke research signify a substantial advancement, enabling the generation of targeted damage with enhanced consistency and less variability relative to conventional models.14-16 This accuracy is especially beneficial for examining areas such as the internal capsule, where little ischemic injury can lead to significant neurological impairments.5,10
The present study introduces a novel method for inducing stroke in the internal capsule of the mouse brain using the photothrombotic method via fiber optic technology. This study represents a step forward in stroke research, providing a robust and innovative model that addresses the limitations of existing methods (Supplementary file 1, Chapter 1).
Methods
Preparation and Anesthesia
An adult male BALB/c mouse (8–12 weeks) weighing 20–22 g was maintained in the Neuroscience Research Center of Tabriz University of Medical Sciences for one week for acclimatization. Before and after the surgical treatment, the mouse was housed in a standard cage under a 12-hour light/dark cycle at 23 ± 1 °C, with unrestricted access to food and water.
To begin the procedure, the animal was anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (9 mg/kg). The depth of anesthesia was monitored by the absence of pedal reflex, and supplemental doses were administered as necessary.
Following shaving skull hair, a longitudinal incision (1.0–1.5 cm) was generated from surface to depth using a surgical blade to expose coronal and sagittal sutures. The interest site (antero-posterior of -1.58 mm, medio-lateral of + 2 mm, and dorso-ventral of -4.5) was targeted using the stereotaxic atlas of Paxinos and Watson.17 Before photothrombotic stroke induction, 150 μg sterile Rose Bengal/g dissolved in normal saline was administered per g/body weight and allowed to disseminate into the blood circulation.18
Photothrombotic Laser Setup and Ischemia Induction
An optical fiber (200 μm core diameter) coupled with a 523 nm laser diode was used to induce ischemia.19 The optic fiber was stereotaxically positioned at the target site, and the device was activated for 10 minutes. This photochemical process involving Rose Bengal resulted in localized thrombosis and ischemia confined to the internal capsule.
Validation of Infarction
Three days post-induction, the mouse was euthanized, and its brain was harvested according to the method suggested by Norouzi-Bonab et al.20 Serial brain sections were prepared and stained with 2,3,5-triphenyl-tetrazolium chloride to visualize and confirm the ischemic lesion (Figure 1) (Supplementary file 1, Chapter 2).18
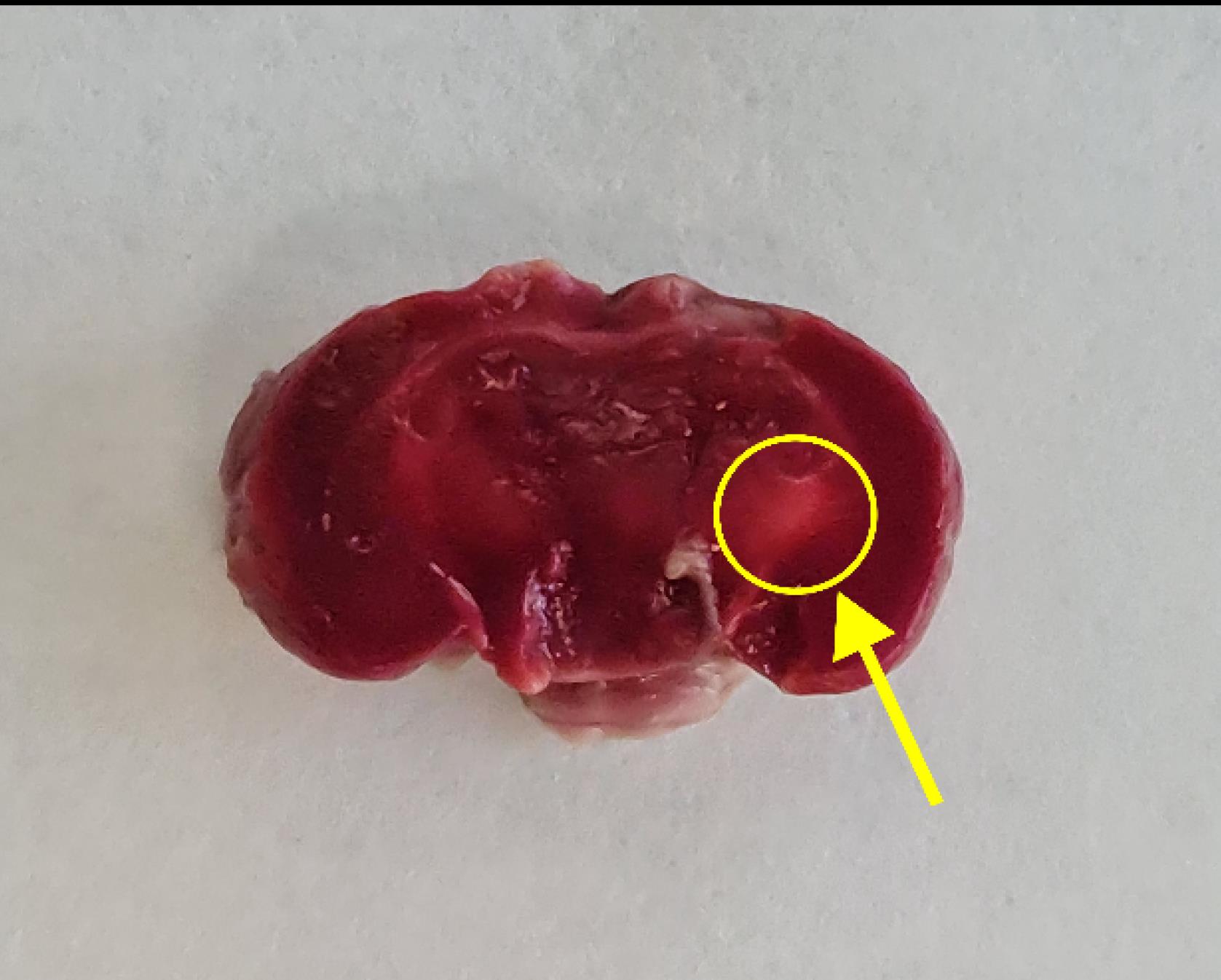
Figure 1.
The Ischemic Lesion Side in the Left Internal Capsule of the Mouse*.Note. *It is shown with a yellow arrow in the encircled area
.
The Ischemic Lesion Side in the Left Internal Capsule of the Mouse*.Note. *It is shown with a yellow arrow in the encircled area
Conclusion
This study has presented an innovative and accurate photothrombotic technique using fiber optic technology for a stroke model that targets the internal capsule in the mouse brain, overcoming the notable limitations of conventional stroke models. Techniques such as middle cerebral artery occlusion or mechanical injury frequently result in inconsistent infarct sizes, non-specific damage, and difficulties in separating effects on tiny, deep brain areas (e.g., the internal capsule). The photothrombotic method utilizing fiber optic technology presents significant advantages, including the capacity to precisely target specific areas, such as the internal capsule, thereby reducing inadvertent harm to adjacent structures and facilitating concentrated investigations of internal capsule ischemia. This approach offers fine control over the time and degree of ischemia damage, enhancing repeatability and dependability across trials. This model enhances the representation of subcortical ischemic strokes, thus bridging the divide between preclinical studies and clinical situations and improving the relevance of findings to human stroke research (Supplementary file 1, Chapter 3).
Authors’ Contribution
Conceptualization: Seyed Zanyar Athari.
Data curation: Faraz Norouzi-Bonab, Mahsa Hasanzadeh-Moghadam, Kimia Motlagh Asghari, Seyed Zanyar Athari.
Formal analysis: Faraz Norouzi-Bonab, Kimia Zabihi.
Funding acquisition: Seyed Zanyar Athari.
Investigation: Faraz Norouzi-Bonab, Seyed Zanyar Athari.
Methodology: Mahsa Hasanzadeh-Moghadam.
Project administration: Mahsa Hasanzadeh-Moghadam, Seyed Zanyar Athari.
Resources: Mahsa Hasanzadeh-Moghadam, Seyed Zanyar Athari.
Software: Faraz Norouzi-Bonab, Kimia Zabihi.
Supervision: Seyed Zanyar Athari.
Validation: Mahsa Hasanzadeh-Moghadam, Seyed Zanyar Athari.
Visualization: Faraz Norouzi-Bonab, Mahsa Hasanzadeh-Moghadam.
Writing–original draft: Faraz Norouzi-Bonab, Kimia Zabihi, Kimia Motlagh Asghari.
Writing–review & editing: Mahsa Hasanzadeh-Moghadam, Seyed Zanyar Athari.
Competing Interests
None.
Supplementary Files
Supplementary file 1. Video-Documented Mouse Model of Internal Capsule Stroke Induced by the Photothrombotic Method Using Fiber Optic Technology: A Novel Approach (Movie).
(mp4)
References
- Feigin VL, Abate MD, Abate YH, Abd ElHafeez S, Abd-Allah F, Abdelalim A. Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol 2024; 23(10):973-1003. doi: 10.1016/s1474-4422(24)00369-7 [Crossref] [ Google Scholar]
- Pu L, Wang L, Zhang R, Zhao T, Jiang Y, Han L. Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke 2023; 54(5):1330-9. doi: 10.1161/strokeaha.122.040073 [Crossref] [ Google Scholar]
- Monsour M, Borlongan CV. The central role of peripheral inflammation in ischemic stroke. J Cereb Blood Flow Metab 2023; 43(5):622-41. doi: 10.1177/0271678x221149509 [Crossref] [ Google Scholar]
- Shehjar F, Maktabi B, Rahman ZA, Bahader GA, James AW, Naqvi A. Stroke: molecular mechanisms and therapies: update on recent developments. Neurochem Int 2023; 162:105458. doi: 10.1016/j.neuint.2022.105458 [Crossref] [ Google Scholar]
- Nabika S, Kiya K, Satoh H, Mizoue T, Oshita J, Kondo H. Ischemia of the internal capsule due to mild head injury in a child. Pediatr Neurosurg 2007; 43(4):312-5. doi: 10.1159/000103313 [Crossref] [ Google Scholar]
- Puig J, Pedraza S, Blasco G, Daunis-I-Estadella J, Prados F, Remollo S. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol 2011; 32(5):857-63. doi: 10.3174/ajnr.A2400 [Crossref] [ Google Scholar]
- Emos MC, Khan Suheb MZ, Agarwal S. Neuroanatomy, internal capsule. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023.
- Freire MA, Lima RR, Bittencourt LO, Guimaraes JS, Falcao D, Gomes-Leal W. Astrocytosis, inflammation, axonal damage and myelin impairment in the internal capsule following striatal ischemic injury. Cells 2023; 12(3):457. doi: 10.3390/cells12030457 [Crossref] [ Google Scholar]
- Li Y, Zhang J. Animal models of stroke. Animal Model Exp Med 2021; 4(3):204-19. doi: 10.1002/ame2.12179 [Crossref] [ Google Scholar]
- Zeng L, Hu S, Zeng L, Chen R, Li H, Yu J. Animal models of ischemic stroke with different forms of middle cerebral artery occlusion. Brain Sci 2023; 13(7):1007. doi: 10.3390/brainsci13071007 [Crossref] [ Google Scholar]
- de Montmollin E, Schwebel C, Dupuis C, Garrouste-Orgeas M, da Silva D, Azoulay E. Life support limitations in mechanically ventilated stroke patients. Crit Care Explor 2021; 3(2):e0341. doi: 10.1097/cce.0000000000000341 [Crossref] [ Google Scholar]
- Seto A, Taylor S, Trudeau D, Swan I, Leung J, Reeson P. Induction of ischemic stroke in awake freely moving mice reveals that isoflurane anesthesia can mask the benefits of a neuroprotection therapy. Front Neuroenergetics 2014; 6:1. doi: 10.3389/fnene.2014.00001 [Crossref] [ Google Scholar]
- Mosneag IE, Flaherty SM, Wykes RC, Allan SM. Stroke and translational research - review of experimental models with a focus on awake ischaemic induction and anaesthesia. Neuroscience 2024; 550:89-101. doi: 10.1016/j.neuroscience.2023.11.034 [Crossref] [ Google Scholar]
- Boyko M, Kuts R, Gruenbaum BF, Tsenter P, Grinshpun J, Frank D. An alternative model of laser-induced stroke in the motor cortex of rats. Biol Proced Online 2019; 21:9. doi: 10.1186/s12575-019-0097-x [Crossref] [ Google Scholar]
- Yamamura K, Kiriu N, Tomura S, Kawauchi S, Murakami K, Sato S. The cause of acute lethality of mice exposed to a laser-induced shock wave to the brainstem. Sci Rep 2022; 12(1):9490. doi: 10.1038/s41598-022-13826-6 [Crossref] [ Google Scholar]
- Kuts R, Melamed I, Shiyntum HN, Gruenbaum BF, Frank D, Knyazer B, et al. Laser-induced brain injury in the motor cortex of rats. J Vis Exp. 2020(163). 10.3791/60928.
- Paxinos G, Franklin KB. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. Cambridge, MA: Academic Press; 2019.
- Shabani Z, Rahbarghazi R, Karimipour M, Ghadiri T, Salehi R, Sadigh-Eteghad S. Transplantation of bioengineered Reelin-loaded PLGA/PEG micelles can accelerate neural tissue regeneration in photothrombotic stroke model of mouse. Bioeng Transl Med 2022; 7(1):e10264. doi: 10.1002/btm2.10264 [Crossref] [ Google Scholar]
- Hosseini SM, Gholami Pourbadie H, Naderi N, Sayyah M, Zibaii MI. Photothrombotically induced unilateral selective hippocampal ischemia in rat. J Pharmacol Toxicol Methods 2018; 94(Pt 1):77-86. doi: 10.1016/j.vascn.2018.06.003 [Crossref] [ Google Scholar]
- Norouzi-Bonab F, Zabihi K, Hasanzadeh-Moghadam M, Athari SZ. Visualized rapid brain extraction in rats. Biomed Res Bull 2023; 1(3):109-12. doi: 10.34172/biomedrb.2023.21 [Crossref] [ Google Scholar]