Biomed Res Bull. 1(1):7-10.
doi: 10.34172/biomedrb.2023.03
Original Article
Investigation of Biochemical Factors in Multiple Sclerosis Patients With Fingolimod and Natalizumab Drugs
Mobina Belalzadeh 1, Farshad Anguti 1, Samira Vedadi 2, Mina Deljavan Ghodrati 1, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Immunology, Faculty of Medicine, Tabriz Branch of Islamic Azad University, Tabriz, Iran
Abstract
Background:
Multiple sclerosis (MS) is a chronic inflammatory disease that demyelinates the central nervous system. Myelin, a two-layer lipid membrane around axons, is responsible for signaling and nerve impulse transmission. Fingolimod as the first oral treatment for MS patients is fingolimod. This drug reduces magnetic resonance imaging (MRI) activity and relapse rate by preventing the migration of T cells from the secondary lymphoid organs into the blood circulation. This study aimed to evaluate biochemical factors in MS patients.
Methods:
A total of 30 MS patients who applied for the MS clinic of Imam Reza hospital (Tabriz-Iran) were chosen for this study. The patients were divided into three groups, including the MS group, MS+Fingolimod, and MS+Natalizumab. For measuring the erythrocyte sedimentation rate (ESR), high-sensitivity C-reactive protein (hs-CRP), and neurofilament light chain (NfL) factors, all samples were thawed, processed, and assayed with immunoassay kits.
Results:
The NfL level decreased for both natalizumab and fingolimod groups compared with the MS group (P<0.0001). Likewise, the ESR level decreased in the fingolimod group more than in the natalizumab group (P<0.0001). In addition, the CRP level decreased in the fingolimod and natalizumab group compared with the MS group (P<0.0001).
Conclusion:
Based on the obtained results it can be concluded that the level of ESR and NfL is high in MS patients, but natalizumab and fingolimod decrease these biochemical factors in MS patients.
Keywords: Multiple sclerosis, Natalizumab, Fingolimod
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease that demyelinates the central nervous system. Myelin, a two-layer lipid membrane around axons, is responsible for signaling and nerve impulse transmission. Myelin protects the axon from harmful external factors and enhances the transmission of electrical impulses. Optic nerves, brain, and spinal cord are involved in MS patients. The most important symptoms of MS include fatigue, numbness, muscle spasms, mobility and vision problems, loss of balance, acute paralysis, tremor, as well as thinking, learning, and planning problems. The first symptoms of MS appear between the ages of 20 and 40. Diagnosing MS is difficult due to the wide variety of its symptoms. Studies show that major histocompatibility complex genes play a role in MS along with environmental factors. The prevalence of MS is increasing in developed and developing countries.1-4 Previously, the only drugs available to treat MS were steroids, but today new compounds have been approved to deal with the course of the disease and improve the individual’s condition. Identifying the best treatment and drug for MS patients can be difficult; accordingly, the clinical presentation of the patient is extremely important for choosing the right treatment. For the relapsing-remitting form of MS, three types of drugs are recommended such as selective immunosuppressive agents, disease-modifying agents, and recently approved drugs.5-7 Natalizumab is the first monoclonal antibody approved for the treatment of MS.8-10 This drug reduces the relapse rate of the disease by preventing lymphocytes from entering the central nervous system.11-15 Progressive multifocal leukoencephalopathy is the main complication of natalizumab treatment.16-19 Moreover, the first oral treatment for MS patients is fingolimod. This drug reduces magnetic resonance imaging (MRI) activity and relapse rate by preventing the migration of T cells from the secondary lymphoid organs into the blood circulation.20-22 Bradycardia, increased risk of infection, leukopenia, increased risk of skin cancer, macular edema, and liver dysfunction are some of the side effects of fingolimod.23 Furthermore, in one clinical trial, a decrease in brain volume was observed in patients who received the drug compared to the placebo.24-28 This study aimed to investigate the expression of the biochemical factors in patients who take MS drugs.
Materials and Methods
Patients and Samples
A total of 30 MS patients who applied for the MS clinic of Imam Reza hospital in Tabriz, Iran, were chosen for this study. The patients were allocated into three groups (n = 10), including the MS group, MS + Fingolimod, and MS + Natalizumab. The 10 cc of whole blood was taken and centrifuged. For measuring the erythrocyte sedimentation rate (ESR), high-sensitivity C-reactive protein (hs-CRP), and neurofilament light chain (NfL) factors, all samples were thawed, processed, and assayed with immunoassay kits. Inclusion criteria were patients who were treated with fingolimod and natalizumab. The exclusion criteria were patients who had a chronic disease such as diabetes and patients who were treated with other MS drugs.
Data Analysis
The GraphPad Prisma 6 software was used for data analysis. The t test was used for statically significant analysis (P < 0.0001) of the data.
Results
The results of NfL levels indicated that NfL levels in MS patients were higher than those in the control group and the drugs group. Moreover, the result showed that natalizumab can decrease the NfL level more compared to fingolimod (Figure 1A). Furthermore, the ESR level in MS patients was high than that in other groups. In addition, the results of the present study indicated no significant difference between the natalizumab and fingolimod groups, but the ESR level in both groups was lower than that in the MS group (Figure 1B). The findings regarding CRP levels also revealed the same results as NfL and ESR levels (Figure 1C).
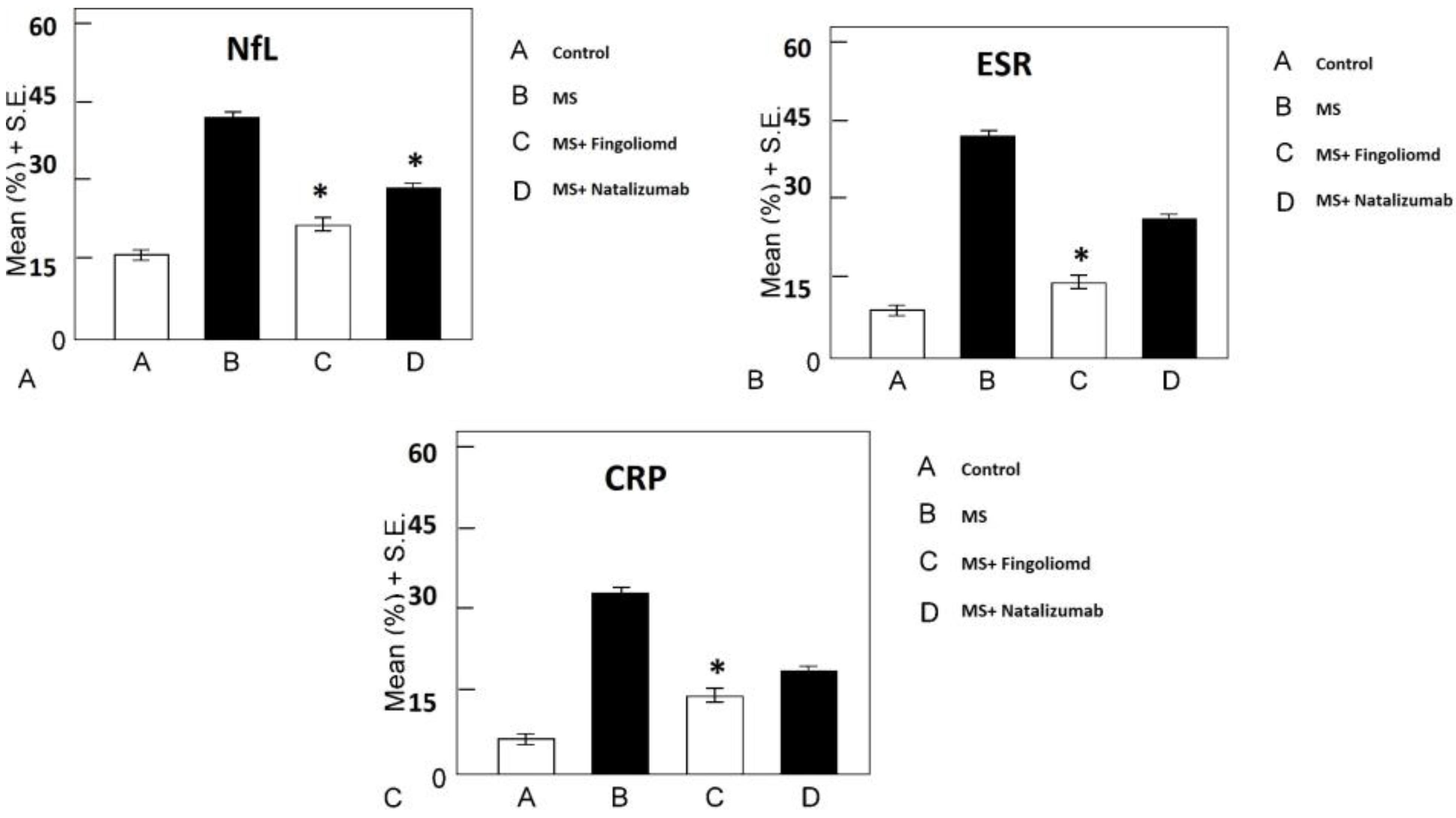
Figure 1.
The Biochemical Marker Levels in MS Patients: (A) The NfL level decreased in the natalizumab and fingolimod groups compared with the MS group (P < 0.0001); (B) The ESR level decreased in the fingolimod group more the than in natalizumab group (P < 0.0001); (C) The CRP level decreased in fingolimod and natalizumab groups compared with MS group (P < 0.0001)
Note. MS: Multiple sclerosis; NfL: Neurofilament light chain; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein.
.
The Biochemical Marker Levels in MS Patients: (A) The NfL level decreased in the natalizumab and fingolimod groups compared with the MS group (P < 0.0001); (B) The ESR level decreased in the fingolimod group more the than in natalizumab group (P < 0.0001); (C) The CRP level decreased in fingolimod and natalizumab groups compared with MS group (P < 0.0001)
Note. MS: Multiple sclerosis; NfL: Neurofilament light chain; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein.
Discussion
As discussed earlier biochemical factors affect brain lipids which are related to a person’s nutrition.29-37 Previous studies have focused on understanding the value of NfL measurements in MS by combining conventional MRI measurements and clinical data with NfL in the blood and/or cerebrospinal fluid.38-46
Another factor that can be effective is nitric oxide. There is a lot of evidence suggesting that nitric oxide can play a role in MS. This substance is also effective in various aspects of MS such as synaptic transmission, inflammation, axonal degeneration, and neuron loss. However, the exact mechanism for this disease has not yet been discovered, and there are many uncertainties in this regard. Further, there is still a long way to prevent damage to neurons through this substance.47-51
The other biochemical factor for MS is uric acid. It is a natural peroxynitrite scavenger, which can be relevant to MS. This chemical is believed to damage myelin and axon of neurons in inflammatory MS lesions. It is believed that the low level of serum uric acid in MS patients is due to the activity of the inflammatory disease and is not related to primary deficiency. Additionally, serum uric acid level can be a good indicator to monitor disease activity. Therefore, increasing the serum level of uric acid can be a suitable measure for MS patients.52
It can be concluded that the level of ESR and NfL is high in MS patients, but natalizumab and fingolimod decrease these biochemical factors in MS patients.
Acknowledgments
The present study was conducted in the Immunology Research Center of Tabriz University of Medical Sciences. Accordingly, the authors thank Imam Reza hospital of Tabriz Medical University.
Authors’ Contribution
Conceptuliazation: Mobina Belalzadeh.
Data Curation:Frashad Anguti.
Formal Analysis: Samira Vedadi.
Investigation:Mina Deljavan Ghodrati.
Methodology: Mobina Belalzadeh, Frahsad Anguti,Samira Vedadi.
Project Adminstration: Mina Deljavan Ghodrati.
Software: Samira Vedadi.
Supervision: Mina Deljavan Ghodrati.
Writing – Original Draft:Samira Vedadi, Mobina Belalzadeh.
Writing – Review & Editing:Mina Deljavan Ghodrati.
Ethical Approval
The study was approved by the the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1401.459).
Competing Interests
None.
References
- Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV. Atlas of Multiple Sclerosis 2013: a growing global problem with widespread inequity. Neurology 2014; 83(11):1022-4. doi: 10.1212/wnl.0000000000000768 [Crossref] [ Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57(2):178-201. doi: 10.1016/j.neuron.2008.01.003 [Crossref] [ Google Scholar]
- Greenfield AL, Hauser SL. B-cell therapy for multiple sclerosis: entering an era. Ann Neurol 2018; 83(1):13-26. doi: 10.1002/ana.25119 [Crossref] [ Google Scholar]
- Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol 2012; 11(2):157-69. doi: 10.1016/s1474-4422(11)70274-5 [Crossref] [ Google Scholar]
- Kakalacheva K, Lünemann JD. Environmental triggers of multiple sclerosis. FEBS Lett 2011; 585(23):3724-9. doi: 10.1016/j.febslet.2011.04.006 [Crossref] [ Google Scholar]
- Chitnis T. Role of puberty in multiple sclerosis risk and course. Clin Immunol 2013; 149(2):192-200. doi: 10.1016/j.clim.2013.03.014 [Crossref] [ Google Scholar]
- Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol 2012; 11(2):157-69. doi: 10.1016/s1474-4422(11)70274-5 [Crossref] [ Google Scholar]
- Alpayci M, Bozan N, Erdem S, Gunes M, Erden M. The possible underlying pathophysiological mechanisms for development of multiple sclerosis in familial Mediterranean fever. Med Hypotheses 2012; 78(6):717-20. doi: 10.1016/j.mehy.2012.02.017 [Crossref] [ Google Scholar]
- Markovic-Plese S, Pinilla C, Martin R. The initiation of the autoimmune response in multiple sclerosis. Clin Neurol Neurosurg 2004; 106(3):218-22. doi: 10.1016/j.clineuro.2004.02.018 [Crossref] [ Google Scholar]
- Tavazzi E, Rovaris M, La Mantia L. Drug therapy for multiple sclerosis. CMAJ 2014; 186(11):833-40. doi: 10.1503/cmaj.130727 [Crossref] [ Google Scholar]
- Giannini M, Portaccio E, Ghezzi A, Hakiki B, Pastò L, Razzolini L. Pregnancy and fetal outcomes after glatiramer acetate exposure in patients with multiple sclerosis: a prospective observational multicentric study. BMC Neurol 2012; 12:124. doi: 10.1186/1471-2377-12-124 [Crossref] [ Google Scholar]
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis European/Canadian Glatiramer Acetate Study Group. Ann Neurol 2001; 49(3):290-7. [ Google Scholar]
- Aharoni R. The mechanism of action of glatiramer acetate in multiple sclerosis and beyond. Autoimmun Rev 2013; 12(5):543-53. doi: 10.1016/j.autrev.2012.09.005 [Crossref] [ Google Scholar]
- Zivadinov R, Reder AT, Filippi M, Minagar A, Stüve O, Lassmann H. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology 2008; 71(2):136-44. doi: 10.1212/01.wnl.0000316810.01120.05 [Crossref] [ Google Scholar]
- Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology. 1993;43(4):655-61. 10.1212/wnl.43.4.655.
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996; 39(3):285-94. doi: 10.1002/ana.410390304 [Crossref] [ Google Scholar]
- Ebers GC. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352(9139):1498-504. doi: 10.1016/s0140-6736(98)03334-0 [Crossref] [ Google Scholar]
- Mellergård J, Edström M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler 2010; 16(2):208-17. doi: 10.1177/1352458509355068 [Crossref] [ Google Scholar]
- Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354(9):899-910. doi: 10.1056/NEJMoa044397 [Crossref] [ Google Scholar]
- Dalton CM, Miszkiel KA, Barker GJ, MacManus DG, Pepple TI, Panzara M. Effect of natalizumab on conversion of gadolinium enhancing lesions to T1 hypointense lesions in relapsing multiple sclerosis. J Neurol 2004; 251(4):407-13. doi: 10.1007/s00415-004-0332-4 [Crossref] [ Google Scholar]
- Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 2010; 9(4):438-46. doi: 10.1016/s1474-4422(10)70028-4 [Crossref] [ Google Scholar]
- Cohen JA, Chun J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann Neurol 2011; 69(5):759-77. doi: 10.1002/ana.22426 [Crossref] [ Google Scholar]
- Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362(5):387-401. doi: 10.1056/NEJMoa0909494 [Crossref] [ Google Scholar]
- Radue EW, O’Connor P, Polman CH, Hohlfeld R, Calabresi P, Selmaj K. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol 2012; 69(10):1259-69. doi: 10.1001/archneurol.2012.1051 [Crossref] [ Google Scholar]
- Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367(12):1098-107. doi: 10.1056/NEJMoa1114287 [Crossref] [ Google Scholar]
- O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365(14):1293-303. doi: 10.1056/NEJMoa1014656 [Crossref] [ Google Scholar]
- Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH. Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359(17):1786-801. doi: 10.1056/NEJMoa0802670 [Crossref] [ Google Scholar]
- Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380(9856):1829-39. doi: 10.1016/s0140-6736(12)61768-1 [Crossref] [ Google Scholar]
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012; 380(9856):1819-28. doi: 10.1016/s0140-6736(12)61769-3 [Crossref] [ Google Scholar]
- US Food and Drug Administration. Peripheral and Central Nervous System Drugs Advisory Committee meeting — Alemtuzumab Advisory Committee background package (BLA103948\5139). Available from: www.fda.gov/downloads/advisorycommittees/committeemeetngmaterals/drugs/ peripher alandcentralnervoussystemdrugadvisorycommittee/ucm374188.pdf. Accessed November 2013.
- Goldberg P. Multiple sclerosis: vitamin D and calcium as environmental determinants of prevalence. Int J Environ Stud 1974; 6(2-3):121-9. doi: 10.1080/00207237408709641 [Crossref] [ Google Scholar]
- Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol 2014; 5:244. doi: 10.3389/fphys.2014.00244 [Crossref] [ Google Scholar]
- Riccio P, Rossano R. Nutrition facts in multiple sclerosis. ASN Neuro 2015; 7(1):1759091414568185. doi: 10.1177/1759091414568185 [Crossref] [ Google Scholar]
- Encinas JM, Manganas L, Enikolopov G. Nitric oxide and multiple sclerosis. Curr Neurol Neurosci Rep 2005; 5(3):232-8. doi: 10.1007/s11910-005-0051-y [Crossref] [ Google Scholar]
- van Rensburg SJ, Kotze MJ, van Toorn R. The conundrum of iron in multiple sclerosis--time for an individualised approach. Metab Brain Dis 2012; 27(3):239-53. doi: 10.1007/s11011-012-9290-1 [Crossref] [ Google Scholar]
- Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol 2002; 1(4):232-41. doi: 10.1016/s1474-4422(02)00102-3 [Crossref] [ Google Scholar]
- Elishkevitz KP, Nussinovitch U, Nussinovitch M. Lactic dehydrogenase isoenzymes in adolescents with multiple sclerosis. Pediatr Neurol 2009; 41(4):259-62. doi: 10.1016/j.pediatrneurol.2009.04.018 [Crossref] [ Google Scholar]
- Koch M, De Keyser J. Uric acid in multiple sclerosis. Neurol Res 2006; 28(3):316-9. doi: 10.1179/016164106x98215 [Crossref] [ Google Scholar]
- Norman AW. The mode of action of vitamin D. Biol Rev Camb Philos Soc 1968; 43(1):97-137. doi: 10.1111/j.1469-185x.1968.tb01111.x [Crossref] [ Google Scholar]
- Thompson VW, Deluca HF. Vitamin D and phospholipid metabolism. J Biol Chem 1964; 239:984-9. [ Google Scholar]
- Hosoya N, Watanable T, Fumimori A. The action of vitamin D in vivo and in vitro on the incorporation of DL-[3-14C] serine into phosphatidyl-serine. Biochim Biophys Acta 1964; 84:770-1. doi: 10.1016/0926-6542(64)90041-1 [Crossref] [ Google Scholar]
- Cruess RL, Clark I. Alterations in the lipids of bone caused by hypervitaminosis A and D. Biochem J 1965; 96(1):262-5. doi: 10.1042/bj0960262 [Crossref] [ Google Scholar]
- Zull JE, Czarnowska-Misztal E, DeLuca HF. On the relationship between vitamin D action and actinomycin-sensitive processes. Proc Natl Acad Sci U S A 1966; 55(1):177-84. doi: 10.1073/pnas.55.1.177 [Crossref] [ Google Scholar]
- Mellanby E. Experimental Rickets (Special Report Series No. 61). London: Medical Research Council; 1921.
- Jones JH. VI Effects of deficiency. The Vitamins 1954; 2:223-32. [ Google Scholar]
- Lewis RW. Temperature and pressure effects on the fatty acids of some marine ectotherms. Comp Biochem Physiol 1962; 6(1):75-89. doi: 10.1016/0010-406x(62)90045-2 [Crossref] [ Google Scholar]
- Eybel CE, Simon RG. Fatty acid composition of the neutral lipids and individual phospholipids of muscle of cold-stressed arctic mice. Lipids 1970; 5(7):590-6. doi: 10.1007/bf02531335 [Crossref] [ Google Scholar]
- Patterson GW. Effect of culture temperature on fatty acid composition of Chlorella sorokiniana. Lipids 1970; 5(7):597-600. doi: 10.1007/bf02531336 [Crossref] [ Google Scholar]
- Ferguson KA, Conner RL, Mallory FB. The effect of ergosterol on the fatty acid composition of Tetrahymena pyriformis. Arch Biochem Biophys 1971; 144(1):448-50. doi: 10.1016/0003-9861(71)90501-7 [Crossref] [ Google Scholar]
- Davison AN. Lipid metabolism in nervous tissue. Comprehensive Biochemistry 1970; 18:293-329. [ Google Scholar]
- Encinas JM, Manganas L, Enikolopov G. Nitric oxide and multiple sclerosis. Curr Neurol Neurosci Rep 2005; 5(3):232-8. doi: 10.1007/s11910-005-0051-y [Crossref] [ Google Scholar]
- Bachmann R, Eugster HP, Frei K, Fontana A, Lassmann H. Impairment of TNF-receptor-1 signaling but not fas signaling diminishes T-cell apoptosis in myelin oligodendrocyte glycoprotein peptide-induced chronic demyelinating autoimmune encephalomyelitis in mice. Am J Pathol 1999; 154(5):1417-22. doi: 10.1016/s0002-9440(10)65395-3 [Crossref] [ Google Scholar]