Biomed Res Bull. 1(3):96-104.
doi: 10.34172/biomedrb.2023.19
Original Article
Gender Differences in Response to Statin Therapy in Ischemic Stroke Patients With SLCO1B1 388A>G Polymorphism: A Clinical Study
Tayebeh Sabokbar 1  , Ehsan Sharifipour 1, *
, Ehsan Sharifipour 1, *  , Masumeh Zamanlu 1
, Masumeh Zamanlu 1  , Tahereh Komeili Movahhed 2
, Tahereh Komeili Movahhed 2  , Mohammad Aghaali 3
, Mohammad Aghaali 3  , Motahare Salarvand 4
, Motahare Salarvand 4  , Fariedoddin Javaherian 5
, Fariedoddin Javaherian 5  , Seyyed Amir Hejazi 1
, Seyyed Amir Hejazi 1 
Author information:
1Neuroscience Research Center, Qom University of Medical Sciences, Qom, Iran
2Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran
3Department of Epidemiology, School of Medicine, Qom University of Medical Sciences, Qom, Iran
4Student Research Committee, School of Pharmacy, Shahid Sadoughi University of Medical Science, Yazd, Iran
5School of Medicine, Qom University of Medical Sciences, Qom, Iran
Abstract
Background:
Statins are widely used for the medical management of vascular conditions, including ischemic stroke. However, genetic factors and polymorphisms, including SLCO1B1 388A>G single-nucleotide polymorphism (SNP), have shown significant effects in response to statin therapy. To the best of our knowledge, gender-gene interaction in response to statin therapy, affected by the SLCO1B1 388 A>G SNP, has not been investigated yet. The current study describes the therapeutic outcomes of this variation in terms of clinical evaluations and laboratory parameters.
Methods:
Seventy patients diagnosed with acute ischemic stroke were recruited for this study. Blood samples were collected. The SLCO1B1 388A>G gene was genotyped using a polymerase chain reaction-restriction fragment length polymorphism method, and the three possible polymorphisms (388GG, 388GA, and 388AA) were detected accordingly. Statin treatment was prescribed at a dose of 40 mg of Atorvastatin or 20 mg of Rosuvastatin daily. Laboratory assessments, including a lipid profile, liver function tests, and lactate dehydrogenase, as well as clinical evaluations, including scores of stroke severity, were obtained at baseline and after a three-month follow-up.
Results:
Iranian ischemic stroke patients showed all three possible polymorphisms, including 30 patients with AA, 28 patients with AG, and 12 patients with GG. Significant SNP variations and gender-gene interactions for most measures of the lipid profile and some clinical trends were found in such a manner that the GG genotype was associated with relevant resistance to statin treatment, while the AA genotype, particularly in male patients, was associated with more response to statin treatment.
Conclusion:
This investigation adds influential gender differences to the previously reported SLCO1B1 388A>G SNP-induced variations of statin therapeutic response in stroke patients.
Keywords: Statin treatment, Statin resistance, Gene-drug interaction, Lipid lowering effect, Gender influence
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Statin treatment is now fundamental management for reducing vascular complications, with well-known benefits for patients with previous ischemic strokes. While reducing the burden of stroke morbidity and mortality is an alarming health priority worldwide, statins are considered an outstanding strategy for primary and secondary prevention of this disease. The main rationale for statin prescription is that they obviously cause clinically positive changes in the lipid profile, particularly cholesterol. However, evidence suggests that further effects occur with statins, and various biological properties of statins are involved in inducing their benefits.1-5 Overall, there are serious uncertainties and controversies about the exact therapeutic mechanisms of statins, their ideal effective doses, their proper target, and their systemic side effects.6
Related details and uncertainties are subject to further studies in order to optimize statin prescriptions. Individual and inter-patient variations are parts of these uncertainties; therefore, genotypes and polymorphisms are increasingly emphasized as contributing to these variations. Single-nucleotide polymorphisms (SNPs) are the most widespread genetic individual variations, reported as having a role in the altered effects of statins. An involving SNP is located in the SLCO1B1 gene, the solute carrier organic anion transporter family member 1B1 gene. This gene encodes the organic anion transfer polypeptide 1B1 (OATP1B1), which is active for the uptake of many endogenous and exogenous compounds, including statins. One of the most common variants of the SLCO1B1 gene is SNP388A > G, which influences the uptake of statins, thereby altering their plasma concentration and consequently their biological activity.7-9 It should be noted that polymorphisms and their related variations in therapeutic effects, in general or particularly for statins, have shown heterogeneous distribution among populations and ethnic groups, while gender differences have received little attention, adding to the considerable gap of information and understanding in this regard.6,9,10
Thereupon, the current investigation aimed at exploring the therapeutic response to statin treatment in Iranian ischemic stroke patients carrying various SLCO1B1 polymorphisms, with regard to their gender. Assessments included both laboratory and clinical findings at baseline and in the three-month follow-up. In general, the results of studying short- and long-term genetic variations in ischemic stroke and statin treatment are anticipated to combine with advanced technology in order to have predictions and optimizations for statin therapy.
Methods
This study is an analytical cohort study.
Sample Size
The sample size was determined using the information provided by Amarenco et al,11 considering the percentage of response to treatment equal to 32% and 11% in the two groups, and values as much as 5% for Alpha and 20% for Beta. According to the sample size formula for comparing percentages, the sample size was calculated as much as 70 subjects.
Subjects
Seventy patients aged 68.59 ± 15.06 years, including 65.7% males (n = 46), were recruited from the inpatient wards of Shahid Beheshti Hospital, a tertiary care academic hospital in Qom, Iran, from April 2017 to October 2018. Acute ischemic stroke was diagnosed by an expert neurologist after complete medical evaluations, including neuroimaging (computed tomography scans and brain magnetic resonance imaging with a diffusion-weighted imaging sequence).
Inclusion Criteria
-
Diagnosis of an ischemic stroke by an expert neurologist
-
Nationality of Iranian
-
No contraindication to statin treatment
-
Age greater than 18 years
Exclusion Criteria
-
Pregnancy
-
Clinical diagnosis of familial hypercholesterolemia
-
Malignancy
-
Renal failure or other kidney diseases
-
Medications affecting the lipid profile (i.e., other lipid-lowering agents or contraceptives)
-
Age under 18
Sample Collection
Samples were collected in accordance with good laboratory practices and ethical codes. Two 12-hour fasting blood samples (10 mL) were obtained at baseline (at the beginning of the study before treatment) and at the three-month follow-up. Plasma concentrations were measured for the lipid profile, including total cholesterol (TC), triglycerides (TG), low-density lipoproteins (LDL), high-density lipoproteins (HDL), liver function parameters, including alanine transaminase, aspartate aminotransferase, alkaline phosphatase (ALK), total bilirubin, and lactate dehydrogenase, by a biochemical analyzer using enzymatic methods.
Clinical Assessments and Treatment
Clinical assessments were obtained at baseline (beginning of the study before treatment) and at the three-month follow-up, including scores of the modified Rankin scale (mRS) and the National Institute of Health Stroke Scale (NIHSS), by an expert neurologist. Statin treatment was prescribed at a dose of 40 mg of Atorvastatin or 20 mg of Rosuvastatin daily. Related treatments and care for ischemic stroke patients were due, according to the highest standards of stroke care that occur in a stroke unit in hospitals capable of running the “stroke code” (as in Shahid Beheshti Hospital of Qom).
Laboratory Assessments
Lipid, Lipoprotein, and Biochemical Measurements
Plasma TC, HDL, and TG concentrations, as well as LDH and liver function tests, were measured using standard enzymatic methods of biochemical assessments with an automated analyzer (Hitachi 917, Hitachi Ltd., Tokyo, Japan) using kits from Boehringer Mannheim (BM, Germany). HDL was measured after phosphotungstic acid and magnesium precipitation. Ultimately, the LDL level was calculated accordingly.
Genomic DNA Analysis
Genomic DNA was extracted from ethylenediaminetetraacetic acid-anticoagulated blood by means of a Thermo Scientific Gene JET whole blood genomic DNA purification mini kit (Thermo Fisher Scientific Inc., USA). SCLO1B1 (388A > G) gene polymorphisms were detected using the polymerase chain reaction–restriction fragment length polymorphism method.7 Genotyping of SLCO1B1 388 A > G (rs2306283) was performed, and the SNP A 274-bp fragment of the SLCO1B1 gene was generated by polymerase chain reaction (PCR) amplification with 60 pmol of each primer [(F): 5̕ -GCA AAT AAA GGG GAA TAT TTC TC3̕ and (R): 5̕ -AGA GAT GTA ATT AAA TGT ATA C-3̕ (BIONEER Corporation, Korea)]. PCR was performed according to the routine protocol described previously,7,12 consisting of an initial denaturation at 95 ˚C for 4 minutes, followed by 37 cycles of denaturation at 94 ˚C for 15 seconds, annealing at 48 ˚C for 15 seconds, and extension at 72 ˚C for 45 seconds. A final 10-minute extension at 75 ˚C was completed. After amplification, the PCR products (274 bp) were digested with the cell-lysis index restriction endonuclease (Thermo Fisher Scientific Inc., USA). The three possible genotypes were the GG homozygote appearing as 155- and 119-bp fragments, the A/G heterozygote with 274-, 155-, and 119-bp fragments, and the AA homozygote with 274-bp fragments.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS, Inc.). A Chi-squared test was used to compare the observed SLCO1B1 genotype frequencies with the expected genotype frequencies based on the Hardy-Weinberg equilibrium. Statistical comparisons among groups carrying the three polymorphisms were performed using the analysis of variance. Continuous and categorical values are expressed as means and standard deviations, as well as counts and percentages, and P values less than 0.05 were considered significant.
Results
Demographic and Clinical Characteristics of Subjects
The demographic and clinical characteristics of the investigated ischemic stroke patients are provided in Table 1. As the analysis demonstrates, age, gender, stroke severity, and history of cardiovascular diseases demonstrate a statistically similar distribution among patients with SLCO1B1 388A > G polymorphisms. Moreover, the genotype frequencies of SLCO1B1 388 A > G polymorphism (the first line of Table 1), equaling the allele frequencies of A = 0.629 and G = 0.371, were consistent with the Hardy–Weinberg equilibrium. This allele frequency resembles the reported frequencies previously for the Caucasian population, approximately as much as 0.30–0.45.10
Table 1.
The Demographic and Clinical Characteristics of the Stroke Patients, Considering All Patients and Divided Groups of Patients With Various SLCO1B1 Polymorphisms
|
Characteristics
|
All Subjects
|
SLCO1B1 388A>G Polymorphisms |
P
Value
|
|
AA
|
AG
|
GG
|
| Number (%) |
70 |
30 (42.9%) |
28 (40.0%) |
12 (17.0%) |
-
|
| Age (years ± SD) |
68.59 ± 15.06 |
66.03 ± 13.15 |
71.32 ± 16.5 |
68.4 ± 16.3 |
0.422 |
| Male (n, %) |
46 (65.7%) |
21 (70%) |
17 (60.7%) |
8 (66.7%) |
0.756 |
| Stroke severity (mean ± SD) |
|
|
|
|
|
| mRS |
3.22 ± 2.17 |
3.1 ± 2.39 |
3.61 ± 1.79 |
2.54 ± 2.46 |
0.369 |
| NIHSS |
10.70 ± 10.22 |
10.03 ± 9.52 |
11.92 ± 10.36 |
9.36 ± 12.17 |
0.705 |
| History of cardiovascular disease (n, %) |
7 (10%) |
3 (10%) |
3 (10.7%) |
1 (8.3%) |
0.974 |
Note. NIHSS: National institute of health stroke scale; mRS: Modified Rankin scale; SD: Standard deviation.
NIHSS is typically divided into three categories, namely, mild (NIHSS ≤ 5), moderate (NIHSS 6–15), and severe (NIHSS ≥ 16). The stroke severity was mild (NIHSS ≤ 5) in 37 patients, moderate (NIHSS 6–15) in 19 patients, and severe (NIHSS ≥ 16) in 14 patients. mRS is the most common score used to measure outcomes in stroke trials. Good outcome following stroke is commonly defined as scores 0–2 and poor outcome as 3–6.13
Plasma Lipid Levels of Patients With SLCO1B1 388A>G Polymorphisms
Figure 1 shows the lipid levels of patients with SLCO1B1 388A > G polymorphisms at baseline and after treatment. No significant difference was found in the baseline lipid levels among patients with the three SLCO1B1 polymorphisms. However, the three-month treatment with statins resulted in a highly significant reduction in most parameters of the lipid profile, including TC, LDL, and HDL, as well as some liver function parameters, including ALK and total bilirubin. The mean TC and TG levels were significantly higher in patients with the GG genotype than those with AG and AA genotypes (P = 0.001 and 0.007, respectively). Total bilirubin and ALK levels were reduced with significant differences among genotypes (P = 026 and P = 0.000, respectively) in a way that patients with the GG genotype demonstrated significantly lower levels. No statistically significant difference was found in other parameters (Table 2).
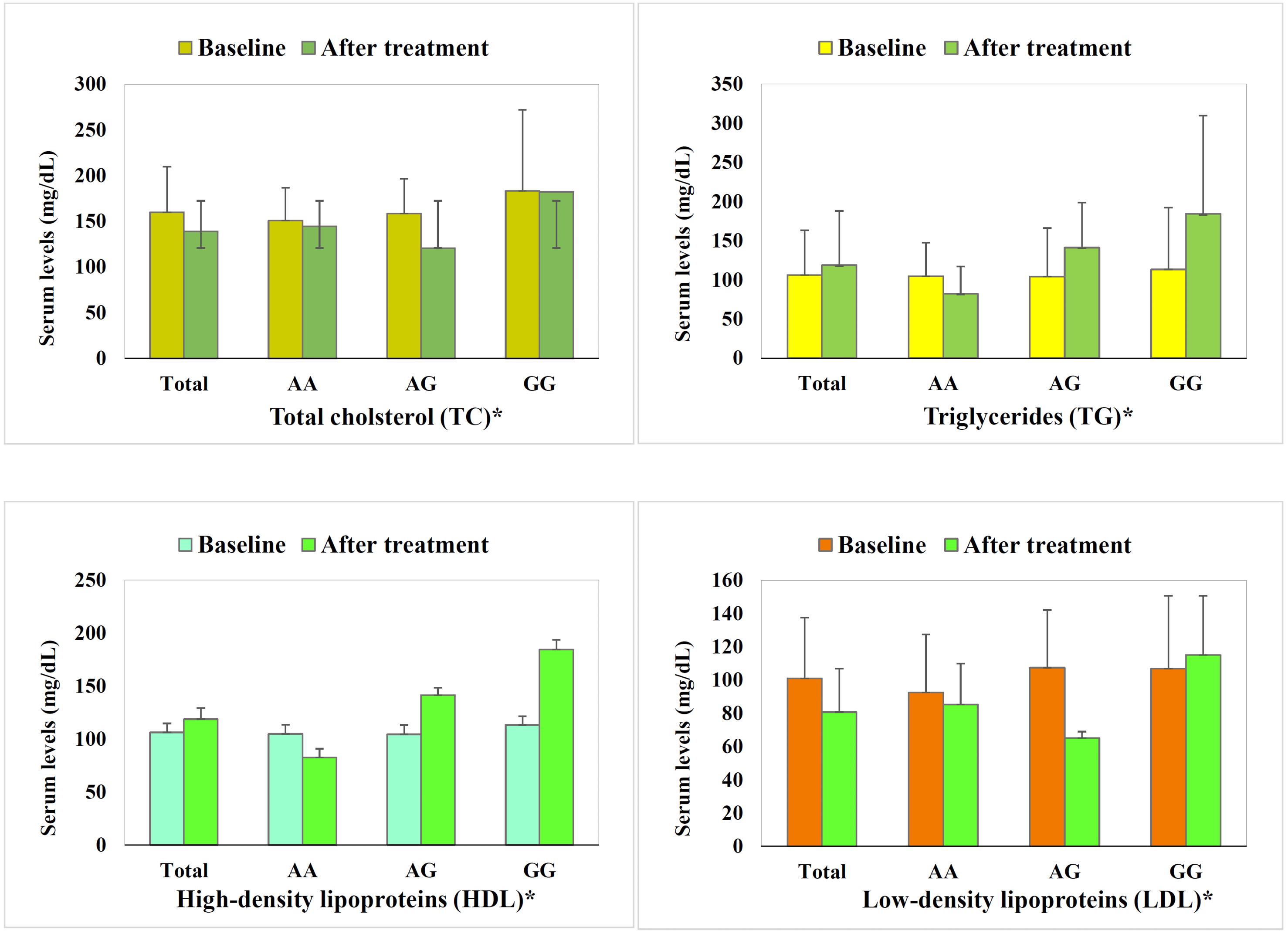

Figure 1.
Lipid Profile of Ischemic Stroke Patients Before Statin Treatment (Baseline) and After Statin Treatment, Illustrated for All Patients (Total), Patients Carrying the AA SLCO1B1 Single-nucleotide Polymorphism, as Well as AG and GG. Note. LDL: Low-density lipoprotein; HDL: High-density lipoprotein; SNP: Single-nucleotide polymorphism.Therapeutic effect variation is demonstrated in a way that AA shows optimal changes in all parameters. AG mostly display optimal changes in parameters, which seem more prominent, including more reductions in total cholesterol (A) and LDL (D), and an increase in HDL (C), yet adverse increase in triglycerides is evident (B). Conversely, GG mostly depicts adverse changes, except for the highest increase in HDL. Baseline lipid profile of patients with various SNPs was statistically similar (P = 0.90-0.16). Statin treatment induced a significant reduction in total cholesterol and LDL, while triglyceride and HDL demonstrated insignificant trends. *All parameters’ changes by statin treatment varied highly significantly among SNPs (P < 0.01).
.
Lipid Profile of Ischemic Stroke Patients Before Statin Treatment (Baseline) and After Statin Treatment, Illustrated for All Patients (Total), Patients Carrying the AA SLCO1B1 Single-nucleotide Polymorphism, as Well as AG and GG. Note. LDL: Low-density lipoprotein; HDL: High-density lipoprotein; SNP: Single-nucleotide polymorphism.Therapeutic effect variation is demonstrated in a way that AA shows optimal changes in all parameters. AG mostly display optimal changes in parameters, which seem more prominent, including more reductions in total cholesterol (A) and LDL (D), and an increase in HDL (C), yet adverse increase in triglycerides is evident (B). Conversely, GG mostly depicts adverse changes, except for the highest increase in HDL. Baseline lipid profile of patients with various SNPs was statistically similar (P = 0.90-0.16). Statin treatment induced a significant reduction in total cholesterol and LDL, while triglyceride and HDL demonstrated insignificant trends. *All parameters’ changes by statin treatment varied highly significantly among SNPs (P < 0.01).
Table 2.
The Plasma Concentration of Parameters of Liver Function in Stroke Patients, Considering All Patients and Divided Groups of Patients With Various SLCO1B1 Polymorphisms
|
Parameters of Liver Function
|
All Subjects
|
SLCO1B1 388A>G Polymorphisms |
P
Valuea Among
|
P
Valuebof Pre/Post
|
|
AA
|
AG
|
GG
|
Polymorphisms
|
Treatment
|
| Alanine transaminase |
|
|
|
|
|
|
| Baseline |
18.73 ± 10.70 |
19.31 ± 11.71 |
18.88 ± 10.23 |
17.00 ± 9.84 |
0.783 |
0.195 |
| After treatment |
24.07 ± 24.63 |
24.37 ± 33.43 |
21.83 ± 9.42 |
35 ± 13.39 |
0.621 |
| Changed by treatment |
4.72 ± 26.45 |
4.40 ± 35.51 |
3.34 ± 13.22 |
14.75 ± 2.217 |
0.733 |
| Aspartate aminotransferase |
|
|
|
|
|
|
| Baseline |
24.02 ± 11.35 |
22.82 ± 9.85 |
26.61 ± 13.82 |
21.33 ± 8.02 |
0.822 |
0.643 |
| After treatment |
23.06 ± 9.01 |
24.2 ± 12.52 |
21.88 ± 2.48 |
22.5 ± 5.8 |
0.669 |
| Changed by treatment |
-0.56 ± 8.68 |
0.74 ± 9.74 |
-2.55 ± 7.76 |
2.5 ± 3.69 |
0.323 |
| Alkaline phosphatase |
|
|
|
|
|
|
| Baseline |
225.52 ± 203.61 |
188.11 ± 117.14 |
284.77 ± 292.07 |
184.42 ± 60.99 |
0.163 |
0.45 |
| After treatment |
194.52 ± 120.82 |
229.32 ± 167.29 |
158.47 ± 20.43 |
185.5 ± 32.77 |
0.125 |
| Changed by treatment |
-11.06 ± 103.61 |
38.25 ± 88.27 |
-71.65 ± 95.63 |
41.5 ± 28.61 |
< 0.001 |
| Lactate dehydrogenase |
|
|
|
|
|
|
| Baseline |
339.05 ± 106.48 |
382.46 ± 83.96 |
421.4 ± 131.43 |
397.33 ± 106.44 |
0.447 |
0.955 |
| After treatment |
387.39 ± 140.39 |
330.82 ± 76.1 |
443.5 ± 172.56 |
392.5 ± 17.68 |
0.025 |
| Changed by treatment |
1.57 ± 174.2 |
-29.68 ± 112.16 |
29.73 ± 226.16 |
31 ± 31.11 |
0.57 |
| Total bilirubin |
|
|
|
|
|
|
| Baseline |
1.11 ± 0.48 |
1.08 ± 0.49 |
1.23 ± 0.5 |
1.08 ± 0.52 |
0.462 |
0.336 |
| After treatment |
1.04 ± 0.38 |
0.8 ± 0.28 |
1.28 ± 0.29 |
1.2 ± 0.25 |
< 0.001 |
| Changed by treatment |
0.08 ± 0.57 |
-0.29 ± 0.57 |
0.09 ± 0.53 |
0.275 ± 0.09 |
0.026 |
Note. ANOVA: Analysis of variance.aBased on ANOVA, bBased on paired t-test.
Statin Therapeutic Response in the Two Genders With SLCO1B1 388A>G Polymorphisms
The three months of statin treatment resulted in variations in the lipid-lowering effect when SLCO1B1 polymorphisms and genders were considered together (Figure 2). Males mostly showed more prominent and more optimal changes in parameters, while females represented some prominent adverse changes, including higher increases in the TC of the GG group (A) and a similar pattern in LDL (D). Generally, the male AA group demonstrated the best response to statin treatment, and the female GG group revealed relative resistance. Though there was no significant difference in response to statin (in terms of lipid profile) among women with SLCO1B1 388A>G polymorphisms, there was a significant difference in TC, LDL, and TG levels in men carrying the G allele in comparison to male AA homozygous patients.
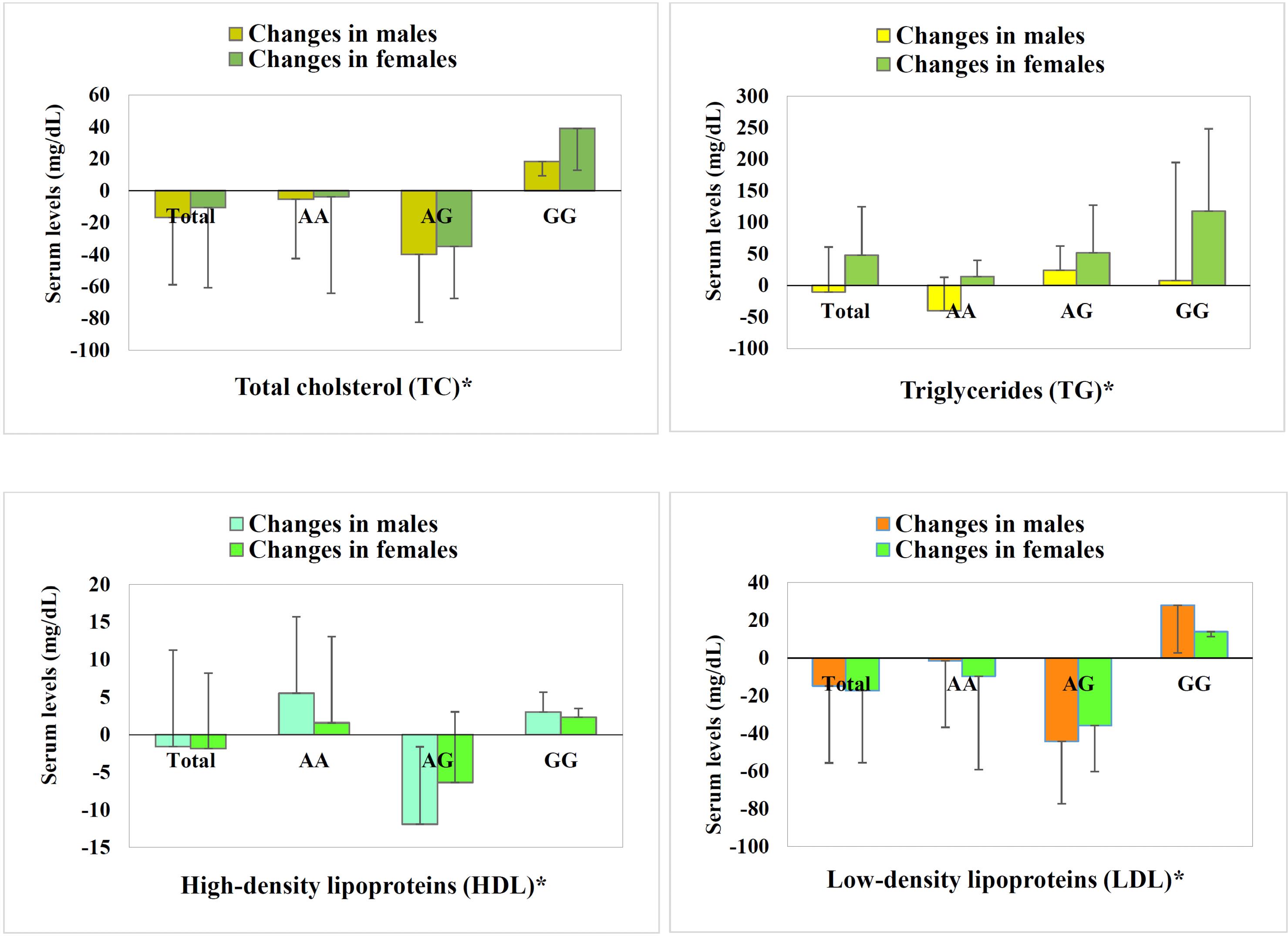
Figure 2.
Lipid Profile of Ischemic Stroke Patients in Males and Females, illustrated for All Patients (Total), Patients Carrying the AA SLCO1B1 Single-nucleotide Polymorphism, as Well as AG and GG. Note. LDL: Low-density lipoprotein; HDL: High-density lipoprotein; SNP: Single-nucleotide polymorphism.Gender differences is demonstrated in a way that males mostly showed more prominent and more optimal changes in parameters, while females represented some prominent adverse changes, including further increases in the total cholesterol of the GG group (A) and a similar pattern in LDL (D). Generally, the male AA group shows the best response to statin treatment, and the female GG group demonstrates relative resistance. *All parameters’ changes by statin treatment in males varied significantly among SNPs (P < 0.05), while insignificant variations in females were shown. Gender differences indicated statistical significance only for triglycerides
.
Lipid Profile of Ischemic Stroke Patients in Males and Females, illustrated for All Patients (Total), Patients Carrying the AA SLCO1B1 Single-nucleotide Polymorphism, as Well as AG and GG. Note. LDL: Low-density lipoprotein; HDL: High-density lipoprotein; SNP: Single-nucleotide polymorphism.Gender differences is demonstrated in a way that males mostly showed more prominent and more optimal changes in parameters, while females represented some prominent adverse changes, including further increases in the total cholesterol of the GG group (A) and a similar pattern in LDL (D). Generally, the male AA group shows the best response to statin treatment, and the female GG group demonstrates relative resistance. *All parameters’ changes by statin treatment in males varied significantly among SNPs (P < 0.05), while insignificant variations in females were shown. Gender differences indicated statistical significance only for triglycerides
Clinical Outcome of Statin Therapy in Stroke Patients With SLCO1B1 388A>G Polymorphisms
Tables 2 and 3 present the stroke severity scores at baseline and after three months of statin treatment in ischemic stroke patients with SLCO1B1 388A > G polymorphisms, indicating higher NIHSS in male patients with GG SNP, though showing marginal significance (P = 0.06). No significant difference was observed in the mRS of patients with SLCO1B1 388A > G polymorphisms.
Table 3.
Baseline Stroke Severity Scores, With Divided Gender Groups of Various SLCO1B1 Polymorphisms
|
Parameters
|
All Subjects
|
SLCO1B1 388A>G |
Polymorphisms
|
P
Valueb
|
P
Valueb
|
|
AA
|
AG
|
GG
|
Among Polymorphisms
|
Among Genders
|
| mRS change |
|
|
|
|
|
|
Male
Female |
-0.89 ± 1.66 |
-1.22 ± 1.86 |
-0.73 ± 1.58 |
0 |
0.379 |
0.259 |
| -1.5 ± 1.78 |
-1.80 ± 2.68 |
-1.50 ± 1.20 |
0 |
0.691 |
|
| NIHSS change |
|
|
|
|
|
|
Male
Female |
-2.31 ± 3.12 |
-2.84 ± 4.09 |
-2.00 ± 1.73 |
-1.00 ± 1.15 |
0.508 |
0.212 |
| -4.57 ± 6.19 |
-7.80 ± 9.86 |
-3.13 ± 1.73 |
0 |
0.336 |
Note. NIHSS: National institute of health stroke scale; mRS: Modified Rankin scale; ANOVA: Analysis of variance.
aBased on ANOVA, bBased on unpaired t-test.
mRS and NIHSS were reduced to 1.8 and 5.9 after three-month statin treatment in all patients, respectively. However, analysis of stroke severity subgroups and polymorphisms was impossible.
The total number of deaths that occurred during the three months of treatment was 9 (12.9%) out of 70 patients. The evaluation of the number of deaths in different SNPs revealed that patients with the GG genotype had a higher percentage (33.3%) than AG genotypes (10.7%) and AA (6.7%), though the difference showed marginal significance.
Discussion
The results of the present study indicated a significant association between SLCO1B1 polymorphisms and the therapeutic response to statins in Iranian ischemic stroke patients, which is interestingly influenced by gender significantly.
The findings of the current study could be considered with a focus on lipid profile parameters as the basic outcome of statin treatment. The lipid profile of the patients after three months of statin therapy represented highly significant optimal changes in most parameters among various polymorphisms. When the findings are sorted according to patients’ gender, the lipid profile parameters after three months among polymorphisms demonstrate differences between the two genders (though the direct gender significant difference is shown only for TG, other parameters - HDL, LDL, and TC - are significantly different for males but not for females, thereby indirectly showing gender significant differences). There are other clues in laboratory parameters, including liver function parameters.
Based on the findings, the GG SLCO1B1 388 genotype was associated with relative resistance to statin treatment, with some tendency toward the female gender. Conversely, the AA genotype, particularly in male patients, represented a more positive prognosis for the statin therapeutic response. Nevertheless, these differences were found to be highly significant in laboratory assessments, with marginal significance in clinical outcomes.
The original concept regards statins as agents for reducing vascular complications, and there is extensive evidence supporting the relevant benefit. However, related details are subject to further studies in order to optimize prescriptions and cover individual and inter-patient variations. Therefore, genotypes and polymorphisms are increasingly investigated to facilitate understanding and improve the outcomes of statin therapy. The general influence of polymorphisms in various study populations has been investigated, raising controversies on many occasions. The gender influence claimed in the current report points to a gap that might seriously deviate from the obtained results. This issue has been neglected in most related studies, and they might need to be re-considered to extract the results that are positively or negatively skewed by gender distribution. Surprisingly, in these investigations, a factor as obvious as gender could be confounding, probably even more than ethnicity or other factors. Such re-analysis might lead to completely novel and contradictory conclusions and, more importantly, approve the gender influence in this regard.
Statins are oral agents that get access to their site of action in hepatocytes through gastrointestinal and hepatocellular transporters. An important factor in the influx transport of statins is the organic anion transfer polypeptide 1B1 (OATP1B1), a polypeptide encoded by the 1B1 member of the solute carrier organic anion transporter family gene (SLCO1B1).7,8 The SLCO1B1 gene is located on chromosome 12p12.2, and non-synonymous SNPs have been identified within this gene, which may affect the transporter activity.2 SNP388A > G is one of the most common variants of this gene that causes an amino acid exchange from asparagine to aspartate at position 130 of the transporter protein.8 Some studies have shown that SNP388A > G exhibits an allelic frequency of approximately 30–45% in the Caucasian population and 60%–90% in Asian populations.10 The current study aimed at a polymorphism claimed to be quite common in the Iranian population, and the frequency obtained in the current results is in line with this claim.
SNP388A > G could be effective on the plasma concentrations of statins and thus on their lipid-lowering activity. Therefore, studying variations of this gene is an important step in understanding the inter-individual differences in response to statins and their side effects (most typically, statin-induced myopathy). The results of the current study indicated SNP388A < G variants affect the ultimate outcome of statin treatment in Iranian ischemic stroke patients; however, gender-gene interaction in this regard shows significant variations in the therapeutic response and tolerance. While some populations have shown no significant differences in the clinical outcome of individuals with SLCO1B1 388A > G polymorphisms, a possible ethnic-gene interaction is also suggested for this SNP (Table 4). As mentioned earlier, the issue of gender influence has been neglected in most related research, which might need to be re-analyzed to extract the results confounded by gender distribution.
Table 4.
The Clinical Outcome in the Stroke Patients After Three Months of Statin Treatment, Considering All Patients and Divided Groups of Patients With Various SLCO1B1 Polymorphisms
|
Parameters
|
All Subjects
|
SLCO1B1 388A>G |
Polymorphisms
|
P
Value
|
P
Value
|
|
AA
|
AG
|
GG
|
Among Polymorphisms
|
Among Genders
|
| Stroke severity scores (mean ± SD) |
|
|
|
|
|
|
| mRS |
|
|
|
|
|
|
Male
Female |
1.89 ± 2.31 |
1.22 ± 1.96 |
2.87 ± 2.45 |
1.25 ± 2.50 |
0.103 |
0.634 |
| 1.57 ± 1.55 |
1.80 ± 1.92 |
1.63 ± 1.41 |
0 |
0.604 |
| NIHSS |
|
|
|
|
|
|
Male
Female |
6.29 ± 8.66 |
4.37 ± 6.55 |
10.13 ± 10.64 |
1.00 ± 2.00 |
0.064
|
0.588 |
| 4.93 ± 5.59 |
6.20 ± 6.06 |
4.75 ± 5.70 |
0 |
0.631 |
| Other complications (n, %) |
|
| Re-current stroke (during study) |
|
Male
Female |
3, 6.5% |
1, 4.8% |
2, 11.8% |
0 |
0.489 |
0.563 |
| 2, 8.3% |
2, 22.2 |
0 |
0 |
0.162 |
| Total |
5, 7.1% |
3, 10% |
2, 7.1% |
0, 0% |
0.524 |
|
| Death (during study) |
|
|
|
|
|
|
Male
Female |
6, 13% |
1, 4.8% |
2, 11.8% |
3, 37.5% |
0.064
|
0.949 |
| 3, 12.9% |
1, 11.1% |
1, 9.1% |
1, 25% |
0.703 |
| Total |
9, 12.9% |
2, 6.7% |
3, 10.7% |
4, 33.3% |
0.06
|
9, 12.9% |
Note. NIHSS: National institute of health stroke scale; mRS: Modified Rankin scale; SD: Standard deviation.
Various reports have demonstrated the association between stroke and lipid disorders, as hypertriglyceridemia is associated with cerebrovascular disease, regardless of the underlying cause, and hypercholesterolemia is associated with transient ischemic stroke.14 It has also been found that in patients with dyslipidemia, especially elevated LDL, the age of the onset of stroke is earlier, and the frequency of ischemic stroke is more likely to be associated with the metabolic syndrome.15,16 The current investigation has focused on the outcome of statin treatment in stroke patients, particularly on the lipid profile of these patients; therefore, the inclination of the current study lies on an evident health priority. There are significant findings for all the mentioned influencing parameters of the lipid profile. Once more, it is reminded that statins are the most potent agents for lipid-lowering treatments.6
In the last decade, the role and mechanism of action of statins related to cerebrovascular events, especially ischemic stroke, have been the focus of attention. Pharmaco-genentic variability has been demonstrated as a determinant and effective factor in the therapeutic response to numerous drugs, induced by inter-individual differences, and statins are no exception. Knowledge about the gene-drug interactions influencing the ultimate therapeutic outcome could lead to more effective therapeutic prescriptions and reduce the side effects of medications, ending with an improved quality of life.6 OATP1B1 is an influx transporter that uptakes statins into hepatocytes. There are 14 non-synonymous SNPs for the SLCO1B1 gene (the OATP1B1-encoding gene), of which only 388A > G and 521T > C could be more frequent than 0.02 and correspondingly are related to changes in the transporter function in the Caucasian population.8,17 Accordingly, the aim of the current investigation was to associate the SLCO1B1 388A > G gene polymorphisms with the therapeutic response of statins in patients with ischemic stroke, importantly presenting the gender-gene interactions.
As per the general therapeutic response of statins in all patients, after 3 months of statin treatment, the plasma levels of lipids revealed a highly significant reduction in TC and LDL. However, the increase in HDL level change in TG did not reach statistical significance. The main mechanism of statins in reducing cholesterol is the reduction of cholesterol biosynthesis through the inhibition of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase enzyme and, consequently, the upregulation of LDL receptors, leading to an increase in the clearance of circulating LDL.18 The three-month follow-up showed an average decrease of 3 units of NIHSS and one unit of mRS, which might partially be influenced by the therapeutic effects of statins. Similarly, it is reported that the beneficial properties of statins, including lipid-lowering effects at the ideal LDL levels ( ≤ 100 mg/dL), improve functional outcomes in patients.19 Moreover, statin has been claimed to prevent recurrent stroke due to its pleiotropic effects, even in patients with normal plasma cholesterol levels, particularly yielding good outcomes of short- and long-term treatment in the acute phase of stroke by their effects on coagulation and fibrinolysis.13 Previous studies have shown that inflammation plays an important role in the pathogenesis of acute stroke and brain damage, and therefore anti-inflammatory effects and the modification of biomarkers and inflammatory mediators such as C-reactive protein and tumor necrosis factor- are among the mechanisms by which statins improve the stroke outcomes.20 Statins also improve neurological outcomes in patients by increasing cerebral blood flow and neuroprotective effects, possibly leading to angiogenesis and synaptogenesis.21
The similar distribution of demographic and clinical characteristics in patients with SLCO1B1 388A > G polymorphisms could point to the fact that the natural grouping of patients according to the SLCO1B1 genotype has not caused a study bias in the basic parameters. It also indicates that the polymorphism apparently does not influence stroke severity at baseline or in the history of cardiovascular disease. Therefore, the influence of this polymorphism has been discussed in the current study based on the changes at the follow-up stage after 3 months of statin treatment. Similarly, comparing parameters of lipid profile and other laboratory assessments at baseline in the patients grouped by SLCO1B1 388A > G polymorphisms yielded no significant differences. However, all parameters of the lipid profile were significantly different at the follow-up, and changes varied among the patients with SLCO1B1 388A > G polymorphisms. Total bilirubin and ALK demonstrated significant differences among the patients as well.
Though the lipid profile is the core outcome of statin therapy, the most practical target for any therapeutic investigation is reflected in the clinical findings. Here, clinical findings point to stroke severity and death rates after three months. The baseline stroke severity in patients with SLCO1B1 388A > G polymorphisms did not differ statistically, but the difference in stroke severity after the three-month follow-up revealed a marginal significance (P = 0.06). It is noteworthy that the data on death rates also show marginal differences among patients with SLCO1B1 388A > G polymorphisms, regardless of gender and in male gender alone. Clinical findings in this research indicated no or little immediate influence of this polymorphism on the severity of the stroke event, while the recovery trend and the three-month outcome might be influenced (showed marginal significance). It is anticipated that similar subjects under more accurate investigations, particularly with longer follow-ups, would yield significant clinical variations.
Overall, the results represented that the outcome differences found between patients with SLCO1B1 388A > G polymorphisms are more pronounced in men, both in terms of clinical evaluations (stroke severity and death rates) and laboratory measures, including lipid profile parameters. These gender differences raise important biological questions about the action mechanisms of drugs in the statin family, which ought to be considered in future investigations. The gender-gene interaction between the SLCO1B1 388A > G polymorphism and statin lipid lowering effect found in this study was in such a manner that after three months of statin treatment, females demonstrated differences in lipid profile among SLCO1B1 388A > G polymorphisms with marginal or no significance, while the AA genotype in male patients was associated with lower LDL and TG (0.042, 0.021, and 0.002). This manner of gender influence is partially approved by the marginally significant difference in the clinical outcome of death in male patients with the AA genotype. To the best of our knowledge, gender-gene interaction in the SLCO1B1 388A > G polymorphism affecting statin therapy has not been reported yet. These findings are anticipated to be combined with advanced technology in the field of personalized medicine to predict and optimize statin therapy.
Conclusion
The findings confirmed that SLCO1B1 gene polymorphism is contributing to the variations in response to statin treatment in ischemic stroke patients, and importantly, there seems to be a gender-geneinteraction. In general, the GG genotype was associated with relative resistance, with some tendency toward the female gender. Conversely, the AA genotype showed a more positive therapeutic prognosis, particularly in male patients.
Differences are found in terms of clinical outcomes and laboratory measures (mainly the lipid profile). These results could differentiate between more statin-resistant and more statin-responsive patients in the future in order to improve therapy outcomes.
Authors’ Contribution
Conceptualization: Tayebeh Sabokbar, Tahereh Komeili Movahhed.
Data curation: Mohammad Aghaali, Motahare Salarvand.
Formal analysis: Fariedoddin Javaherian.
Funding acquisition: Seyyed Amir Hejazi.
Investigation: Seyyed Amir Hejazi.
Methodology: Masumeh Zamanlu.
Project administration: Masumeh Zamanlu.
Resources: Seyyed Amir Hejazi.
Software: Mohammad Aghaali, Motahare Salarvand.
Supervision: Ehsan Sharifipour.
Writing–original draft: Mohammad Aghaali, Motahare Salarvand.
Writing–review & editing: Ehsan Sharifipour.
Competing Interests
None.
Ethical Approval
This project was approved by the Ethics Committee of Qom University of Medical Sciences (Code IR.MUQ.1396.120). The investigation was performed in accordance with international and Iranian ethical codes for clinical research. Written informed consent was obtained from each participant.
Funding
None.
References
- Tachibana-Iimori R, Tabara Y, Kusuhara H, Kohara K, Kawamoto R, Nakura J. Effect of genetic polymorphism of OATP-C (SLCO1B1) on lipid-lowering response to HMG-CoA reductase inhibitors. Drug Metab Pharmacokinet 2004; 19(5):375-80. doi: 10.2133/dmpk.19.375 [Crossref] [ Google Scholar]
- Shabana MF, Mishriki AA, Issac MS, Bakhoum SW. Do MDR1 and SLCO1B1 polymorphisms influence the therapeutic response to atorvastatin? A study on a cohort of Egyptian patients with hypercholesterolemia. Mol Diagn Ther 2013; 17(5):299-309. doi: 10.1007/s40291-013-0038-3 [Crossref] [ Google Scholar]
- O’Regan C, Wu P, Arora P, Perri D, Mills EJ. Statin therapy in stroke prevention: a meta-analysis involving 121,000 patients. Am J Med 2008; 121(1):24-33. doi: 10.1016/j.amjmed.2007.06.033 [Crossref] [ Google Scholar]
- Ma YB, Chan P, Zhang Y, Tomlinson B, Liu Z. Evaluating the efficacy and safety of atorvastatin + ezetimibe in a fixed-dose combination for the treatment of hypercholesterolemia. Expert Opin Pharmacother 2019; 20(8):917-28. doi: 10.1080/14656566.2019.1594776 [Crossref] [ Google Scholar]
- Jung M, Lee S. Effects of statin therapy on the risk of intracerebral hemorrhage in Korean patients with hyperlipidemia. Pharmacotherapy 2019; 39(2):129-39. doi: 10.1002/phar.2211 [Crossref] [ Google Scholar]
- Wechsler LR. Statins and stroke - it’s complicated. N Engl J Med 2020; 382(1):81-2. doi: 10.1056/NEJMe1914757 [Crossref] [ Google Scholar]
- Pasanen MK, Backman JT, Neuvonen PJ, Niemi M. Frequencies of single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide 1B1 SLCO1B1 gene in a Finnish population. Eur J Clin Pharmacol 2006; 62(6):409-15. doi: 10.1007/s00228-006-0123-1 [Crossref] [ Google Scholar]
- Igel M, Arnold KA, Niemi M, Hofmann U, Schwab M, Lütjohann D. Impact of the SLCO1B1 polymorphism on the pharmacokinetics and lipid-lowering efficacy of multiple-dose pravastatin. Clin Pharmacol Ther 2006; 79(5):419-26. doi: 10.1016/j.clpt.2006.01.010 [Crossref] [ Google Scholar]
- Kansu B, Lang D. Genetic polymorphisms as predictive markers for statin therapy: a route to improved cardiovascular patient outcomes?. Bioscie Horiz 2017; 10:hzx011. doi: 10.1093/biohorizons/hzx010 [Crossref] [ Google Scholar]
- Lam YW, Scott SR. Pharmacogenomics: Challenges and Opportunities in Therapeutic Implementation. Academic Press; 2018.
- Amarenco P, Labreuche J, Lavallée P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke 2004; 35(12):2902-9. doi: 10.1161/01.STR.0000147965.52712.fa [Crossref] [ Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther 2003; 304(1):223-8. doi: 10.1124/jpet.102.043026 [Crossref] [ Google Scholar]
- Cappellari M, Deluca C, Tinazzi M, Tomelleri G, Carletti M, Fiaschi A. Does statin in the acute phase of ischemic stroke improve outcome after intravenous thrombolysis? A retrospective study. J Neurol Sci 2011; 308(1-2):128-34. doi: 10.1016/j.jns.2011.05.026 [Crossref] [ Google Scholar]
- Laloux P, Galanti L, Jamart J. Lipids in ischemic stroke subtypes. Acta Neurol Belg 2004; 104(1):13-9. [ Google Scholar]
- Nomura E, Kohriyama T, Matsumoto M, Kobayashi S. Clinical characteristics of first-ever atherothrombotic infarction or lacunar infarction with hyperlipidemia (J-STARS-C): an analysis of data from the stroke data bank of Japan. Intern Med 2005; 44(12):1252-7. doi: 10.2169/internalmedicine.44.1252 [Crossref] [ Google Scholar]
- Matz K, Tatschl C, Sebek K, Dachenhausen A, Brainin M. Dyslipidemia, elevated LDL cholesterol and reduced nocturnal blood pressure dipping denote lacunar strokes occurring during nighttime. Eur J Neurol 2004; 11(11):742-8. doi: 10.1111/j.1468-1331.2004.00811.x [Crossref] [ Google Scholar]
- Romaine SP, Bailey KM, Hall AS, Balmforth AJ. The influence of SLCO1B1 (OATP1B1) gene polymorphisms on response to statin therapy. Pharmacogenomics J 2010; 10(1):1-11. doi: 10.1038/tpj.2009.54 [Crossref] [ Google Scholar]
- Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med 2001; 5(4):378-87. doi: 10.1111/j.1582-4934.2001.tb00172.x [Crossref] [ Google Scholar]
- Stead LG, Vaidyanathan L, Kumar G, Bellolio MF, Brown RD Jr, Suravaram S. Statins in ischemic stroke: just low-density lipoprotein lowering or more?. J Stroke Cerebrovasc Dis 2009; 18(2):124-7. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.016 [Crossref] [ Google Scholar]
- Lakhan SE, Bagchi S, Hofer M. Statins and clinical outcome of acute ischemic stroke: a systematic review. Int Arch Med 2010; 3:22. doi: 10.1186/1755-7682-3-22 [Crossref] [ Google Scholar]
- Restrepo L, Bang OY, Ovbiagele B, Ali L, Kim D, Liebeskind DS. Impact of hyperlipidemia and statins on ischemic stroke outcomes after intra-arterial fibrinolysis and percutaneous mechanical embolectomy. Cerebrovasc Dis 2009; 28(4):384-90. doi: 10.1159/000235625 [Crossref] [ Google Scholar]