Biomed Res Bull. 1(3):92-95.
doi: 10.34172/biomedrb.2023.18
Original Article
Anticancer Effects of Hesperidin on Gastric Cancer Cell Lines and Fibroblast Cell Lines by Reducing the Activation of PI3K Pathway
Jia Tong 1  , Liu Lifang 1, Zheng Dong 1, Zheifeng Xu 1, *
, Liu Lifang 1, Zheng Dong 1, Zheifeng Xu 1, * 
Author information:
1Department of Immunology, Zhejiang University, Zhejiang, China
Abstract
Background:
Cancer is one of the leading causes of death worldwide. In recent years, the need to pay attention to medicinal plants has increased due to the side effects of drugs and drug treatment. Hesperidin has medicinal uses in traditional medicine and is used as an antidote for therapeutic diseases. This study was conducted to investigate the toxicity effect of Hesperidin on gastric cancer cell lines (AGS) and normal fibroblast cell lines (SKM).
Methods:
Hesperidin plant extract with different types (100, 50, 10, and 50 μg/mL) was released on AGS and SKM cell lines for 24 hours by MTT test. The amount of apoptosis induction was evaluated by flow cytometry with Annexin V/PI staining and changes in the expression of genes (BAX and BCL-2) by real-time polymerase chain reaction. The effect of the drug on phosphoinositide 3-kinases (PI3K) was determined by the Western blot technique. The data were analyzed by SPSS statistical software using a one-way variance test and Tukey’s test (P<005).
Results:
Hesperidin had a cytotoxic effect on SKM and AGS cell lines and induced apoptosis. In the treatment with Hesperidin (100 μg/mL) for 24 hours, gene expression decreased in AGS and SKM and increased in BCL2 and BAX, (P<005). The growth rate of PI3K was decreased by the effect of the drug.
Conclusion:
Hesperidin, with a specific effect on SKM and AGS cancer cells, has the ability to grow these cells at a dose of 100 micrograms per milliliter.
Keywords: Hesperidin, Gastric, PI3K
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Gastrointestinal cancers account for about 20% of all existing cancers,1 and among them, stomach cancer is one of the deadliest cancers,2 which is the fourth most common cancer in the world and has a high mortality rate. Approximately 700 000 people die of cancer per year, and stomach cancer is the second leading cause of death after lung cancer.3-6 On the other hand, colon or colorectal cancer, which is also known as intestinal cancer, is caused by the uncontrolled growth of cells in the colon, rectum, or appendix. This cancer is known as one of the most important diseases of the digestive system. Every year, about 40 000 people in the world die due to this disease.7 In recent years, scientists have used plants or some compounds derived from plants to treat diseases because these compounds, in addition to being effective in treating the disease and affecting the rate of cell death,8 have fewer side effects than chemical drugs. Chemical analyses have shown that there are a wide variety of biologically active compounds, such as tannins, polysaccharides, and flavonoids, in plants. In addition, one of the most serious problems with the drugs used in the fight against cancer is that healthy cells are heavily affected by chemical drugs and get damaged.9-12 On the other hand, several genes are involved in the process of apoptosis or programmed cell death, and the stimulation of the expression of pro-apoptotic genes or inhibition of anti-apoptotic genes depends on the type of cell and the stimulus.13 The two main pathways of apoptosis are the extrinsic pathway, or are dependent on death receptors, and the intrinsic pathway, or the mitochondrial pathway.14-17 On the other hand, tumor cells use different strategies to limit the process of apoptosis. Tumors can inhibit apoptosis by increasing the expression of anti-apoptotic regulators (BCL2 and Bcl-XL) or reducing apoptotic factors (BIM, BAX, and PUMA).18-20 Further, in recent years, the use of medicinal plants has become popular.18
Hesperidin is a flavanone glycoside found in citrus fruits. Its aglycone is Hesperetin. Its name is derived from the word “hesperidium”, for fruit produced by citrus trees. Hesperidin was first isolated in 1828 by French chemist M. Lebreton from the white inner layer of citrus peels.19 This study aimed to evaluate the effects of the flavonoid family (Hesperidin) on cancer cell lines.
Materials and Methods
Hesperidin Preparation and Extraction
Hesperidin powder was dissolved in 300 cc of 70% ethanol (hydroalcoholic extract) and 300 cc of distilled water and placed on a shaker at medium speed for 72 hours at room temperature. Next, the extracts were filtered with the help of filter paper. To separate the solvent, a rotary device was used at a temperature of 45 °C. Then, to completely separate the solvent from the extract, the samples were placed inside it for 48 hours at a temperature of 45 °C. Finally, the obtained extracts were dried and kept at -20 °C until use in the laboratory process.
Cell Culture and 3-[4,5-dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide (MTT) Test
Gastric cancer cells (AGS) and fibroblast cells (SKM) cultured in a flask were purchased from Shanghai Institutes for Biological Sciences (SHnaghai, China). AGS cancer cells were cultured in the medium, and normal fibroblast cells were cultured in minimum essential culture medium containing 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 μg/mL), and incubated in a humid incubator (BINDER, Germany) at a temperature of 37 degrees Celsius and with 5% CO2. To perform the MTT test, 1 × 106 cells were cultured in each well of a 96-well plate. The cells were divided into different treatment and control groups. Three wells were allocated to each group, and the experiment was repeated three times. In the treatment groups, 5 concentrations of each of the aqueous and hydroalcoholic extracts (100, 50, and 10 μg/mL) were added to the cells. After incubation for 24 hours, 20 microliters of MTT manufactured by Abcam Company with a concentration of 5 mg/mL were added to each well and incubated for another 4 hours in the dark. Then, the medium on the cells was carefully removed, and 100 μL of dimethyl sulphoxide were added to the house plate. After about 20 minutes, the optical absorbance of each well was read using an enzyme-linked immunosorbent assay reader at a wavelength of 570 nm. The concentration of the extract that inhibits cell growth by 50% was considered the half-maximal inhibitory concentration, and the survival rate of cells was calculated.
Real-Time Quantitative Polymerase Chain Reaction
Based on the results of the MTT test, to measure the change in gene expression of BAX and BCL-2, gastric cancer cells (AGS) and fibroblast cells (SKM) were treated with the Hesperidin extract at a concentration of 100 μg/mL for 24 hours. RNA extraction was performed from cell samples. After RNA extraction, spectrometry and electrophoresis on a 1% agarose gel were used to determine its quantity and quality. The prepared cDNA was kept at -20 °C. RT-qPCR in 45 cycles and a volume of 15 µL with the presence of 1 µL of cDNA, 1 µL of each of the primers (Table 1), 5.7 µL of SYBR-Green single test kit solution, and 5.4 µL of water sterile deionization were used in the device (3000 Corbett-Real time ROTOR GENE), and the beta-actin gene was utilized as an internal control gene. The primers are provided in Table 1.
Table 1.
The Information of Primers
|
Primers
|
Sequence
|
| BAX |
Forward 3’AAGGGACTTACCCATTGGGATAC5’
Revise 3’CTTAAAGGGAATTGGATACCCCG5’ |
| BCL-2 |
Forward 3’GACTGGGATATACCCAAGGATTC5’
Revise 3’AACTTACCAGGGGCATTCATAGG5’ |
| B-actin |
Forward 3’ATTGAAGGGACTTGGATACACCC5’
Revise 3’CTTAAAGGATTGGACGATACCCG5’ |
Determination of the Amount of Programmed Cell Death
According to the results of the MTT test to estimate the amount of apoptosis, gastric cancer cell lines (AGS) and fibroblast cells (SKM) were treated with the extract at a concentration of 100 micrograms for 24 hours. They were placed per milliliter. The category of treated and untreated cells (control) was checked using the Annexin/PI method with a detection kit (BD Kit I PharmingenTM, USA) and with the help of a flow cytometry device (CYFlow, Partec GmbH, Germany). After incubation, the cells were separated by trypsin and neutralized with FBS, and then the cells were centrifuged at 1000 rpm for 5 minutes at a temperature of 4 °C. Next, 200 μL of 10% binding buffer solution were added to the cells, and they were centrifuged at 1000 rpm for 5 minutes. Subsequently, 5 microliters of Annexin V dye were added, and the solution was incubated for 15 minutes at room temperature. The cells were washed with the binding solution, and in the next step, 10 μL of the PI dye were added to the cells. At the end of the analysis, the amount of cell death was measured by the flow cytometry device.
Western Blot
To measure the amount of PI3K protein, the Western blot technique was evaluated on cells treated with sodium dodecyl-sulfate gel according to the protocol using the ANTI-PI3K antibody. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control.
Statically Analysis
Data were analyzed using SPSS Statistics software (version 19) and presented as the mean ± standard deviation (SD) for at least three independently performed experiments, unless otherwise stated. A P value < 0.005 was considered to be statistically significant.
Results
The results obtained from this study showed that Hesperidin (P < 0.005), at a concentration of 100 μg/mL, could significantly induce apoptosis in gastric cancer cells and fibroblasts (Figure 1). The results of gene expression analysis using the RT-qPCR technique demonstrated that the expression of the BCL-2 gene in AGS and SKM cell lines with treatment with a concentration of 100 μg/mL of Hesperidin in 24 hours significantly decreased compared to the beta-actin control gene (Figure 2, P < 0.001), and BAX gene expression was significantly increased in comparison to the beta-actin control gene (P < 0.001); thus, the ratio of BAX/BCL-2 was also increased in cancer samples (SKM-AGS). Data are shown as the mean ± SD. Hesperidin decreased the survival rate of AGS and SKM cells by more than 50% in 24 hours. The results presented in flow cytometry include the percentage of living cells, necrosis, and apoptosis (primary and secondary apoptosis) for cells treated with Hesperidin at a concentration of 5 μg/mL for 24 hours (Table 2). Western blot results revealed that the amount of PI3K protein decreased in both cells at a concentration of 100 μg/mL Hesperidin (Figure 3).
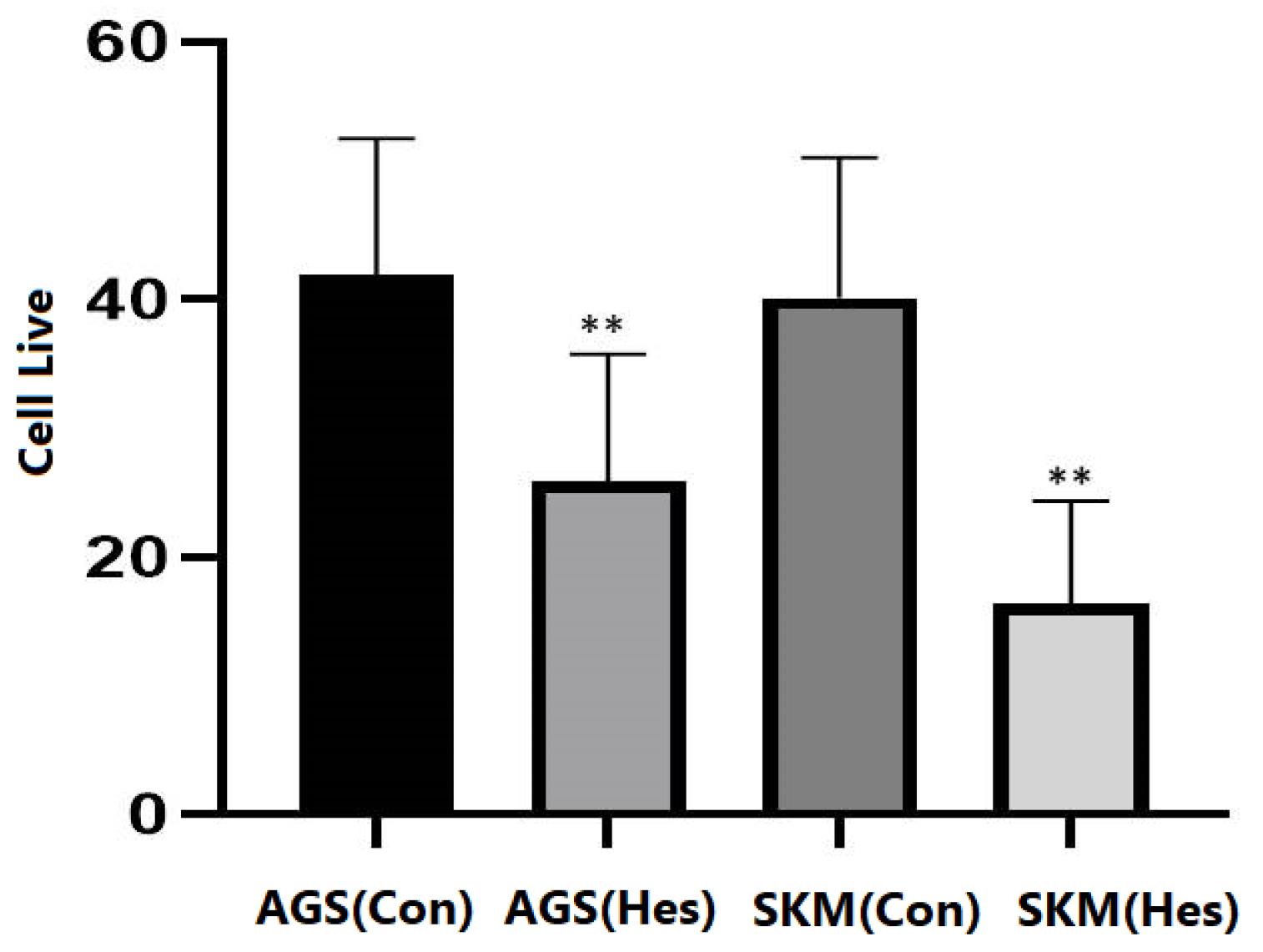
Figure 1.
Cell Live Evaluation
.
Cell Live Evaluation
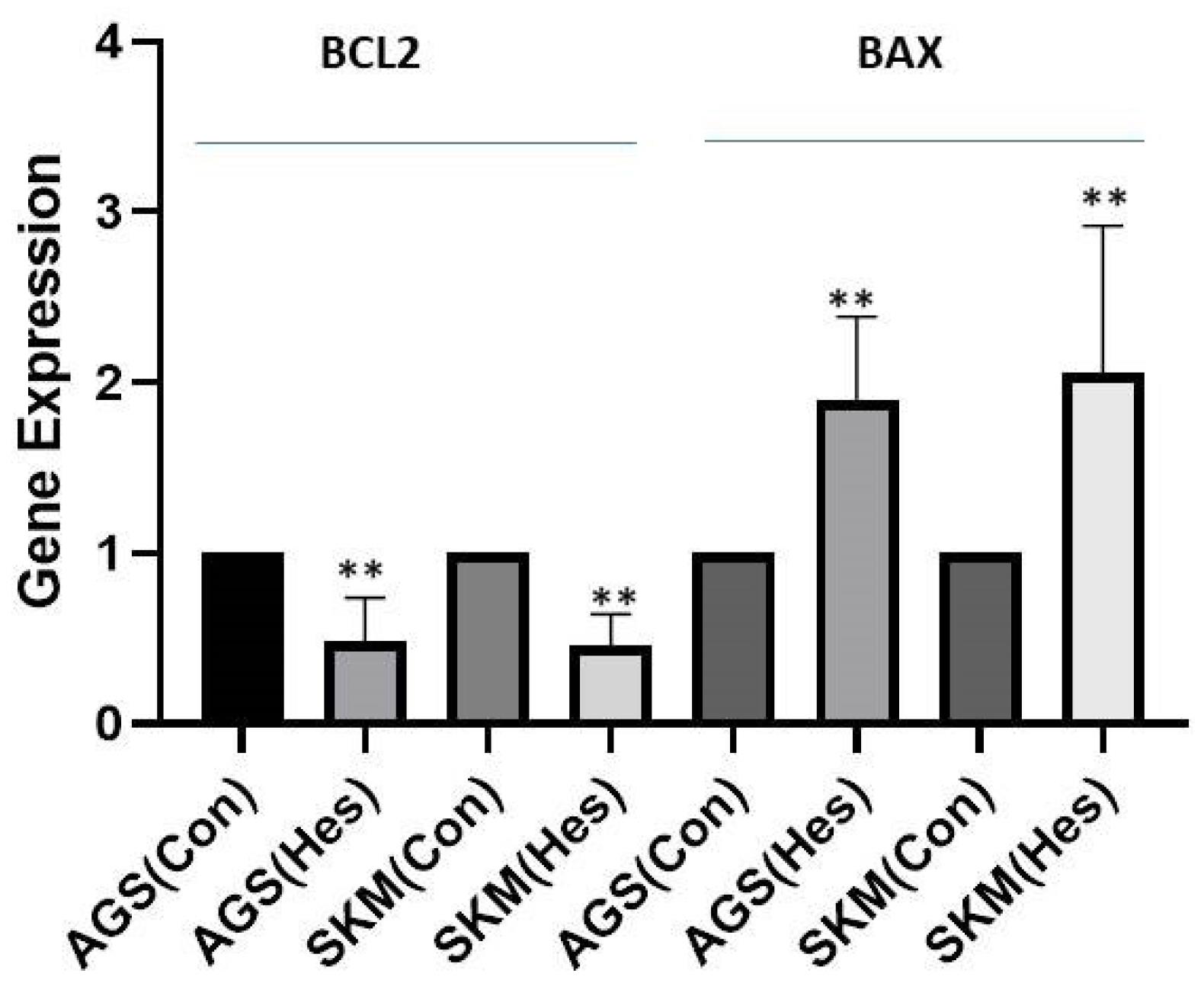
Figure 2.
BCL-2 and BAX Gene Expression
.
BCL-2 and BAX Gene Expression
Table 2.
Flow Cytometry Results for SKM and AGS Cells
|
|
Live Cells
|
Necrosis
|
Apoptosis
|
| SKM |
93.10 |
1.09 |
2.7 |
| AGS |
91.18 |
1.10 |
2.6 |
Note. AGS: Gastric cancer cell line; SKM: Normal fibroblast cell line.
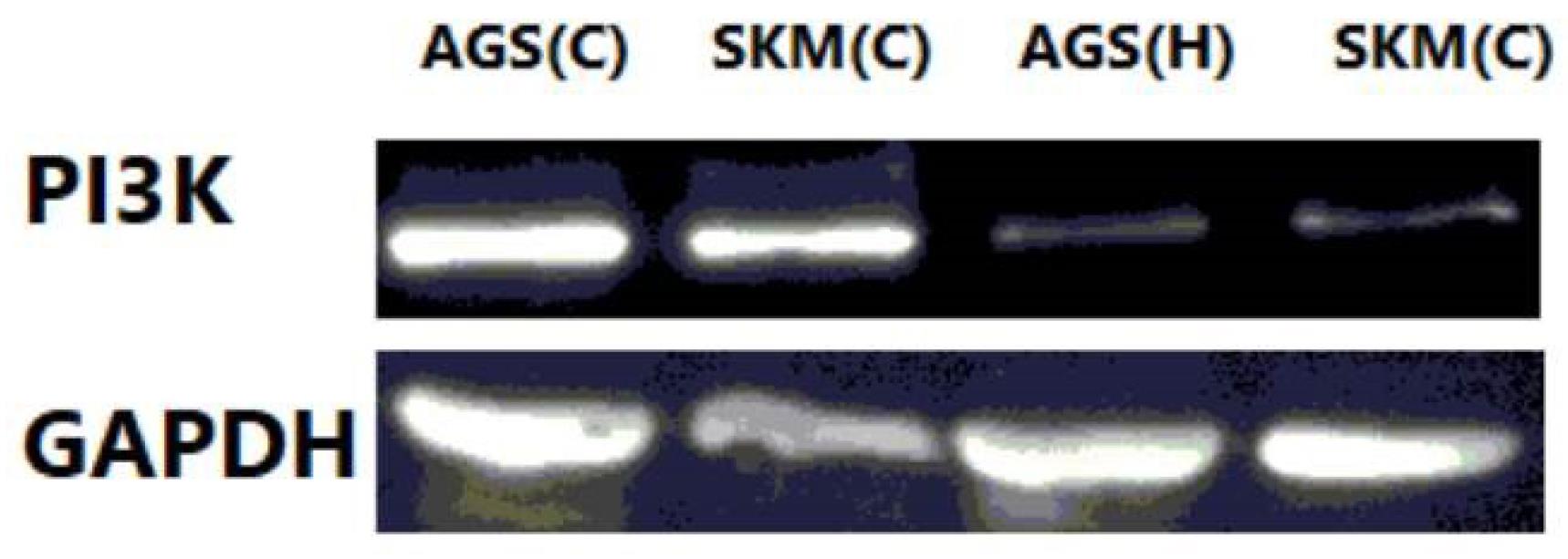
Figure 3.
Western Blot Analysis of PI3K. Note. PI3K: Phosphoinositide 3-kinases
.
Western Blot Analysis of PI3K. Note. PI3K: Phosphoinositide 3-kinases
Discussion
The high prevalence of cancer in the world has drawn the attention of researchers to the need for drugs with fewer side effects and better therapeutic effects,19 so that today, many anti-cancer compounds for the treatment of cancer patients are obtained from plant, marine, and micro-organism sources. Many herbal plants also have extensive therapeutic properties, such as preventing cancer, preventing tumor formation, and treating wounds and infections. In the current research, due to the importance of Hesperidin, attention has been paid to investigating the anti-cancer effects of this plant. Research has shown that the phenolic compounds in plants have anticancer properties,20 and the Hesperidin used in this study also caused a decrease in the population of cancer cells, which could be due to its phenolic compounds. Many studies have been performed on Hesperidin and flavonoids.21 In a study that was conducted, it was determined that Hesperidin is an anti-inflammatory agent for the treatment of K562 human blood myeloid cell cancer, although it does not affect cell proliferation or apoptosis in K562 cells.22 The amount of phenol in Hesperidin is one of the most important compounds that can have anti-cancer properties. In addition, many studies have been conducted on the effect of various plant compounds on gastrointestinal cancers. One study evaluated the effects of Hesperidin on proliferation and apoptosis in human gastric cancer (AGS) cells, and it was found that the survival and migration of AGS cells decreased significantly in a dose-dependent manner after the administration of hesperidin.23
The amount of death observed in cancer cells at high concentrations of Hesperidin used in this research can be justified because phenolic compounds at high concentrations cause damage to body cells and tissues, although it is possible that the same substance at high concentrations has less adverse effects on the cellular structure. According to the obtained results, the effect of Hesperidin on healthy cells was extremely less than on cancer cells, and this compound can be used alone or in combination with anticancer drugs, so that at the same time as the destruction of cancer cells, healthy cells will experience less damage. In this study, it was shown that Hesperidin could induce programmed death in gastric cancer cells by changing the expression levels of BAX and BCL-2 genes. Based on the results, Hesperidin had more cytotoxic effects on AGS cell lines. In general, this effect can be related to the phenolic compounds present in this plant; hence, this plant can be used in its entirety or the compounds derived from it (e.g., phenolic compounds and flavonoids) to reduce the number of gastric cancer cells.
Conclusion
Hesperidin, with a specific effect on SKM and AGS cancer cells, has the ability to grow these cells at a dose of 100 micrograms per milliliter.
Authors’ Contribution
Conceptualization: Jia Tong, Liu Lifang, Zheng Dong.
Data curation: Liu Lifang, Zheng Dong.
Formal analysis: Jia Tong, Zheng Dong.
Funding acquisition: Zheifeng Xu.
Investigation: Jia Tong.
Methodology: Zheng Dong.
Project administration: Zheng Dong.
Resources: Jia Tong.
Software: Jia Tong.
Supervision: Zheifeng Xu.
Validation: Zheifeng Xu.
Writing–original draft: Jia Tong, Liu Lifang, Zheng Dong.
Writing–review & editing: Zheng Dong.
Competing Interests
All of the authors declare that they have no conflict of interests.
Ethical Approval
The ethical approved by Zhejiang university committee from the National Natural Science Foundation of China (Ethical number: 81264236321).
Funding
None.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022; 72(1):7-33. doi: 10.3322/caac.21708 [Crossref] [ Google Scholar]
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12(4):278-87. doi: 10.1038/nrc3236 [Crossref] [ Google Scholar]
- Wu S, Zhu W, Thompson P, Hannun YA. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun 2018; 9(1):3490. doi: 10.1038/s41467-018-05467-z [Crossref] [ Google Scholar]
- Ames BN. Mutagenesis and carcinogenesis: endogenous and exogenous factors. Environ Mol Mutagen 1989; 14 Suppl 16:66-77. doi: 10.1002/em.2850140614 [Crossref] [ Google Scholar]
- Hussain SP, Harris CC. Molecular epidemiology and carcinogenesis: endogenous and exogenous carcinogens. Mutat Res 2000; 462(2-3):311-22. doi: 10.1016/s1383-5742(00)00015-6 [Crossref] [ Google Scholar]
- Rahmani AH, Aldebasi YH. Ficus carica and its constituents role in management of diseases. Asian J Pharm Clin Res 2017; 10(6):49-53. doi: 10.22159/ajpcr.2017.v10i6.17832 [Crossref] [ Google Scholar]
- Almatroodi SA, Khan AA, Aloliqi AA, Syed MA, Rahmani AH. Therapeutic potential of Tamarixaphylla in the prevention of lung injury through the regulation of inflammation, oxidative stress and cell-signaling molecules. Appl Sci 2022; 12(19):9925. doi: 10.3390/app12199925 [Crossref] [ Google Scholar]
- Almatroodi SA, Alnuqaydan AM, Babiker AY, Almogbel MA, Khan AA, Husain Rahmani A. 6-Gingerol, a bioactive compound of ginger attenuates renal damage in streptozotocin-induced diabetic rats by regulating the oxidative stress and inflammation. Pharmaceutics 2021; 13(3):317. doi: 10.3390/pharmaceutics13030317 [Crossref] [ Google Scholar]
- Rahmani AH, Alsahli MA, Khan AA, Almatroodi SA. Quercetin, a plant flavonol attenuates diabetic complications, renal tissue damage, renal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Metabolites 2023; 13(1):130. doi: 10.3390/metabo13010130 [Crossref] [ Google Scholar]
- Almatroodi SA, Almatroudi A, Alsahli MA, Khan AA, Rahmani AH. Thymoquinone, an active compound of Nigella sativa: role in prevention and treatment of cancer. Curr Pharm Biotechnol 2020; 21(11):1028-41. doi: 10.2174/1389201021666200416092743 [Crossref] [ Google Scholar]
- Almatroodi SA, Alsahli MA, Almatroudi A, Rahmani AH. Garlic and its active compounds: a potential candidate in the prevention of cancer by modulating various cell signalling pathways. Anticancer Agents Med Chem 2019; 19(11):1314-24. doi: 10.2174/1871520619666190409100955 [Crossref] [ Google Scholar]
- Rahmani AH, Alsahli MA, Almatroudi A, Almogbel MA, Khan AA, Anwar S. The potential role of apigenin in cancer prevention and treatment. Molecules 2022; 27(18):6051. doi: 10.3390/molecules27186051 [Crossref] [ Google Scholar]
- Almatroodi SA, M AA, A SMA, Alhumaydhi FA, Babiker AY, Khan AA. Potential therapeutic targets of resveratrol, a plant polyphenol, and its role in the therapy of various types of cancer. Molecules 2022; 27(9):2665. doi: 10.3390/molecules27092665 [Crossref] [ Google Scholar]
- Verri WA Jr, Vicentini FT, Baracat MM, Georgetti SR, Cardoso RD, Cunha TM. Flavonoids as anti-inflammatory and analgesic drugs: mechanisms of action and perspectives in the development of pharmaceutical forms. Stud Nat Prod Chem 2012; 36:297-330. doi: 10.1016/b978-0-444-53836-9.00026-8 [Crossref] [ Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 1998; 56(11):317-33. doi: 10.1111/j.1753-4887.1998.tb01670.x [Crossref] [ Google Scholar]
- Garg A, Garg S, Zaneveld LJ, Singla AK. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res 2001; 15(8):655-69. doi: 10.1002/ptr.1074 [Crossref] [ Google Scholar]
- Bhalla N, Dakwale R. Chemotaxonomy of Indigofera Linn. J Indian Bot Soc 1978; 57(2):180-5. [ Google Scholar]
- Pawłowska L. Flavonoids in the leaves of polish species of the genus Betula L. l. The flavonoids of B. pendula Roth. and B. obscura Kot. leaves. Acta Soc Bot Pol 1980; 49(3):281-96. doi: 10.5586/asbp.1980.025 [Crossref] [ Google Scholar]
- Kokkalou E, Kapetanidis I. Flavonoids of the aerial parts of Acinossuaveolens. Pharm Acta Helv 1988; 636:170-3. [ Google Scholar]
- Tsai YF, Chen YR, Chen JP, Tang Y, Yang KC. Effect of hesperidin on anti-inflammation and cellular antioxidant capacity in hydrogen peroxide-stimulated human articular chondrocytes. Process Biochem 2019; 85:175-84. doi: 10.1016/j.procbio.2019.07.014 [Crossref] [ Google Scholar]
- Pandey P, Khan F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr Res 2021; 92:21-31. doi: 10.1016/j.nutres.2021.05.011 [Crossref] [ Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140(6):883-99. doi: 10.1016/j.cell.2010.01.025 [Crossref] [ Google Scholar]
- Pandya PH, Murray ME, Pollok KE, Renbarger JL. The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res 2016; 2016:4273943. doi: 10.1155/2016/4273943 [Crossref] [ Google Scholar]