Biomed Res Bull. 1(4):141-147.
doi: 10.34172/biomedrb.2023.27
Review Article
Effects of Sacituzumab on Breast Cancer: Target Therapy
Elina Armani Khatibi 1  , Tooba Gholikhani 1, 2, *
, Tooba Gholikhani 1, 2, *  , Balam Jimenez Brito 3, Nastaran Farshbaf Moghimi 4
, Balam Jimenez Brito 3, Nastaran Farshbaf Moghimi 4
Author information:
1Student Research Committee, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
2Nanora Pharmaceuticals Ltd, Tabriz, Iran
3Victoria University of Wellington, School of Biological Sciences, Wellington, New Zealand
4Department of Pharmaceutics, School of Pharmacy, Ardabil University of Medical Science, Ardabil, Iran
Abstract
Triple-negative breast cancer (TNBC) is considered one of the most aggressive forms of BC, which increases the risk of cancer-associated death. Despite the availability of specifically targeted medications for treating TNBC with HER2-positive or hormone receptor-positive cancers, chemotherapy is still the mainstay of treatment. Sacituzumab, an antibody-drug conjugate (ADC), targets tumor-associated calcium signal transducer 2 (Trop-2)-expressing cells and presents biologically active metabolites of irinotecan hydrochloride (SN-38). This review evaluates the effectiveness and safety of Sacituzumab in treating metastatic or previously treated TNBC in patients. According to the National Center for Biotechnology Information website, another epithelial metastasis has been included in data on clinical trials and sacituzumab. Sacituzumab has hopeful antitumor effects on patients with metastatic TNBC treated with at least two treatment lines, according to a clinical trial that is in phase I/II. Sacituzumab has a controllable adverse effect, with neutropenia, nausea, and diarrhea being the most frequent negative side effects. As a result of these promising early efficacy data, Sacituzumab’s activity may develop in TNBC, as well as various other epithelial tumors, such as hormone receptor-positive BC.
Keywords: Metastatic breast cancer, Sacituzumab, TNBC, Trop-2, Antibody-drug conjugates
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Triple-negative breast cancer (TNBC) does not display any presence of estrogen or progesterone receptors, and the human epidermal growth factor receptor 2 does not appear to be amplified in this type of cancer.1 Despite being more common in premenopausal women, adversary mutation carriers of the BC gene (BRCA), and African and American women, around 15% of BCs are classified as TNBC.2,3 BC promotion and malignant face can be associated with TNBC, with an elevated risk of recurrence and poor medical consequences.4 TNBC patients have an average total survival of less than 20 months, while the average improvement-free survival remains about 4–5 months with reasonable cytotoxic medications such as chemotherapy.5-9 Generally. Molecular sequencing results have improved the concept of homozygosity of molecular NBC in an approach to determining genomic types related to drug reactions.
There is an overlap between TNBC and intrinsic basal-like subtypes; nearly 60%–78% of TNBCs are basal-like, and about 82% of basal-like cancers are TNBCs.10 There are some genomic sets within TNBC, including BL1 and BL2 as basal-like 1 and 2, luminal androgen receptor (LAR), immunoregulatory, mesenchymal-stem-like, mesenchymal (M), and an unstable cluster.11,12 In some case studies, tumor-infiltrating lymphocytes and mesenchymal cells serving tumors were detected in cancerous tissues, and expression templates were transformed into LAR, M, BL2, and BL1. The type of BL1 was related to an elevated pathologic perfect reaction level in individuals treated with taxane- and platinum-based neo-adjuvant medicine.13,14 The mRNA level profiling data of TNBC tumors has identified four TNBC subtypes, including mesenchymal, basal-like immunosuppressed, LAR, and basal-like immune-activated (BLIA).15,16 Importantly, BLIAs had positive results concerning cancer-free survival.15 More research must be undertaken to assess these types and determine genomic labels that predict reactions to medications. Multiple studies (clinical trials) are being conducted to evaluate medicines in the genomic subsets of TNBC, such as suppressors of androgen receptors in TNBC, which are favorable for AR.10,17 Programmed cell death-1/programmed cell death ligand-1 (PD-1/PD-L1) suppressors have been associated with lasting medical treatment in TNBC patients by targeting antitumor immunity.18 In TNBC, PD-L1 mRNA is found mainly in tumors filtered into immune cells other than cancerous cells.19,20 The Food and Drug Administration (FDA) has approved atezolizumab and nab-paclitaxel for patients with PD-L1-positive, malignant TNBC based on the Impassion 130 trial.21,22 In the Impassion 130 clinical trial, patients were randomly assigned to receive either atezolizumab with nab-paclitaxel or a placebo with nab-paclitaxel. The study had two initial goals. Overall survival (OS in the ITT population and case of a significant finding in the PD-L1-positive subgroup), progression-free survival (PFS in the intention-to-treat [ITT] population and PD-L1 positive subgroup), and PD-L1 mRNA level were determined on tumor-infiltrating immune cells using immunohistochemistry. A PD-L1-positive result was determined by staining of ≥ 1%.
The use of atezolizumab elevated the average PFS from 4.9 to 6.8 months (hazard ratio [HR] = 0.78, 92% CI = 0.71–0.88, P= 0.003) in patients with ITT and from 4.9 to 6.8 months (HR = 0.59, 93% CI = 0.51–0.81, P< 0.002) in patients expressing PD-L1. By adding atezolizumab, the average OS increased from 18.1 months to 20.9 months (HR = 0.79, 91% CI = 0.71–1.13, P= 0.06) in the patients with ITT and from 14.8 to 24.9 months in patients with PD-L1 expression (HR = 0.59, 92% CI = 0.42–0.83), but these findings were not considerably remarkable. Atezolizumab was the first immunotherapy approved for malignant TNBC. However, several clinical trials determined other medication therapies.18 In addition to the current confirmation of atezolizumab in combination with nab-paclitaxel, sequential chemotherapy remains the best option for malignant TNBC. Additionally, sequential single elements are chosen before combinations; however, combination medications could be utilized to treat metastatic cancer.23 Medications such as eribulin, anthracyclines, taxanes, gemcitabine, capecitabine, and navelbine were recommended in this regard.24,25 Individuals with BRCA mutations, poly (ADP-ribose) polymerase (PARP) suppressors, and platinum salts are active.26,27 In the initial raw, the average PFS with palliative chemotherapy is 1.5–4 months without the applied agent.7 This describes the requirement for more systemic medications with better effectiveness in treating patients with malignant TNBC.25,28 In this article, the team of scientists attempts pharmacodynamics to explain the effect of Sacituzumab on malignant TNBC and other epithelial tumors by targeting trop-2 due to reducing the adverse effects of other drugs commonly used in the first line of therapy. The team also aims at investigating the pharmacodynamic, pharmacokinetic, and side effects of Sacituzumab.
Tumor-associated Calcium Signal Transducer 2 Targeting in Triple-negative Breast Cancer
Mode of Operation
A calcium transducer, “trophoblast cell-surface antigen 2 (Trop-2)”, acts as a transmission tract. Various epithelial tumors, such as BC, overexpress Trop-2 in comparison to joint tissues 29,30. Trop-2 has been reported to modulate some signaling pathways. Several signaling pathways are involved in cancer cells’ migration and proliferation.31 Trop-2 expression is observed in 82% of TNBC, and the highest expression is related to a more metastatic cancer period in multiple cancers. This includes breast tumors.30,32 Therefore, the potential of Trop-2 and other epithelial tumors that express Trop-2 as a medical target for TNBC is quite promising. Specific antibody-drug conjugation is called Sacituzumab govitecan (IMMU-132) made of a humanized monoclonal RS7 IgG1κTrop-2 antibody with SN-38 as chemical linkage (Figure 1).33-35 An antibody–drug conjugate (ADC) adoptively confers cytotoxic chemotherapy agents on tumors while inhibiting the growth of healthy tissues 36. This results in an enhanced medical representative with increased intra-tumor concentrations of drugs and reduced toxicities that spread through the body. Recently, ADCs were confirmed for other types of cancer, such as brentuximab for lymphoma, gemtuzumab for acute myeloid leukemia, and other lymphoma types. In addition, inotuzumab is used for acute lymphoblastic leukemia and other diseases in medical development, and ado-trastuzumab has been used for malignant HER2-positive BC.36,37
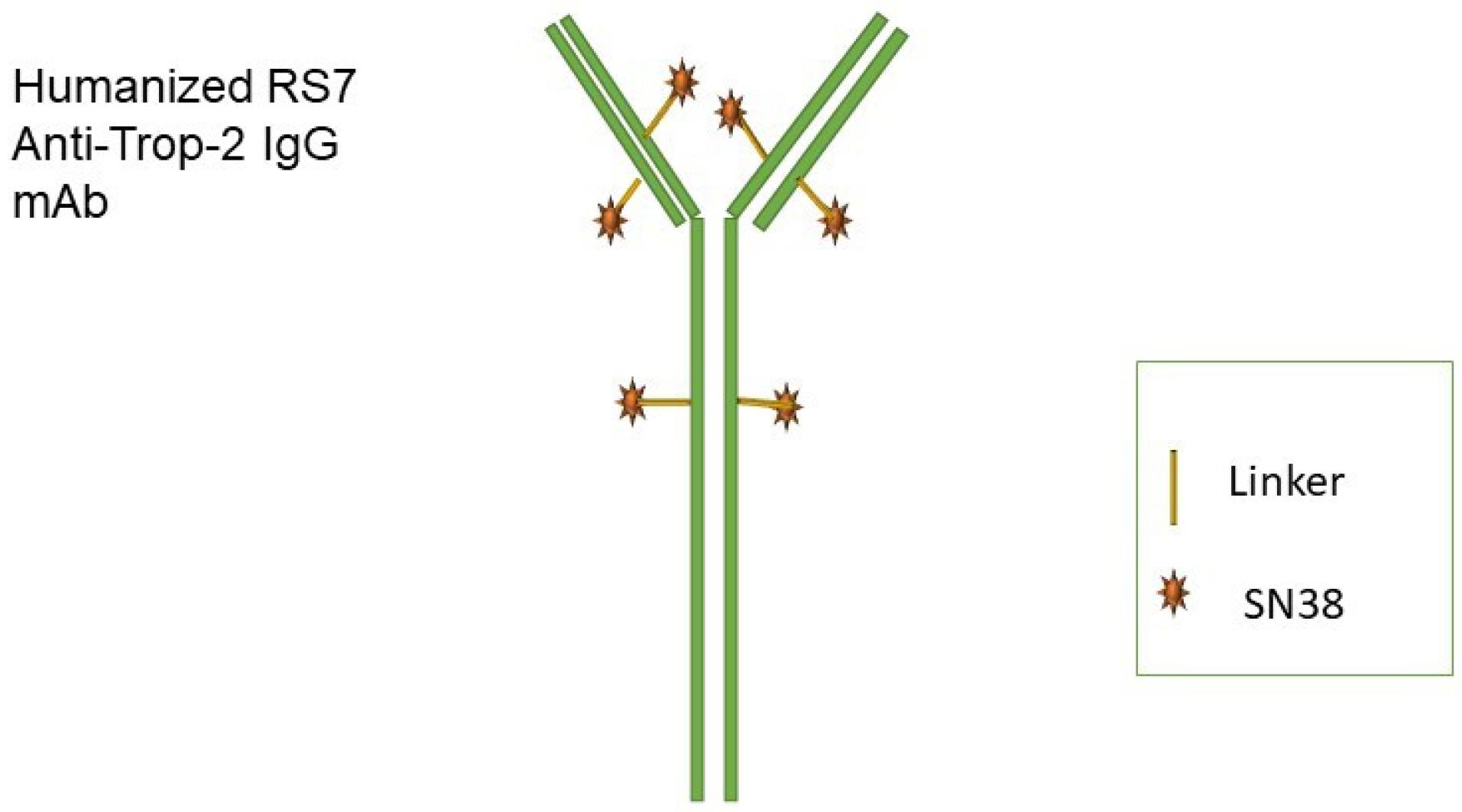
Figure 1.
Sacituzumab Structure. mAb: monoclonal antibody
.
Sacituzumab Structure. mAb: monoclonal antibody
Sacituzumab leads to the internalization of the drug when it binds to Trop-2 within tumors. This, in turn, enables the intracellular delivery of SN-38 (Figure 2).38,39 The unique characteristics of this substance include a high ratio of drug to antibody and a linker known as CL2A, which can be easily broken down.33,40 Linkers with intermediate durability enable SN-38 secretion in Sacituzumab-bound and cancer cells. SN-38 concentrations at the site of action and conjugate binding may enhance the anticancer action of medications. Irinotecan, a topoisomerase-I suppressor, produces an active metabolite called SN-38 with an enhanced potency of about 1000-fold in inducing DNA breaks.41,42
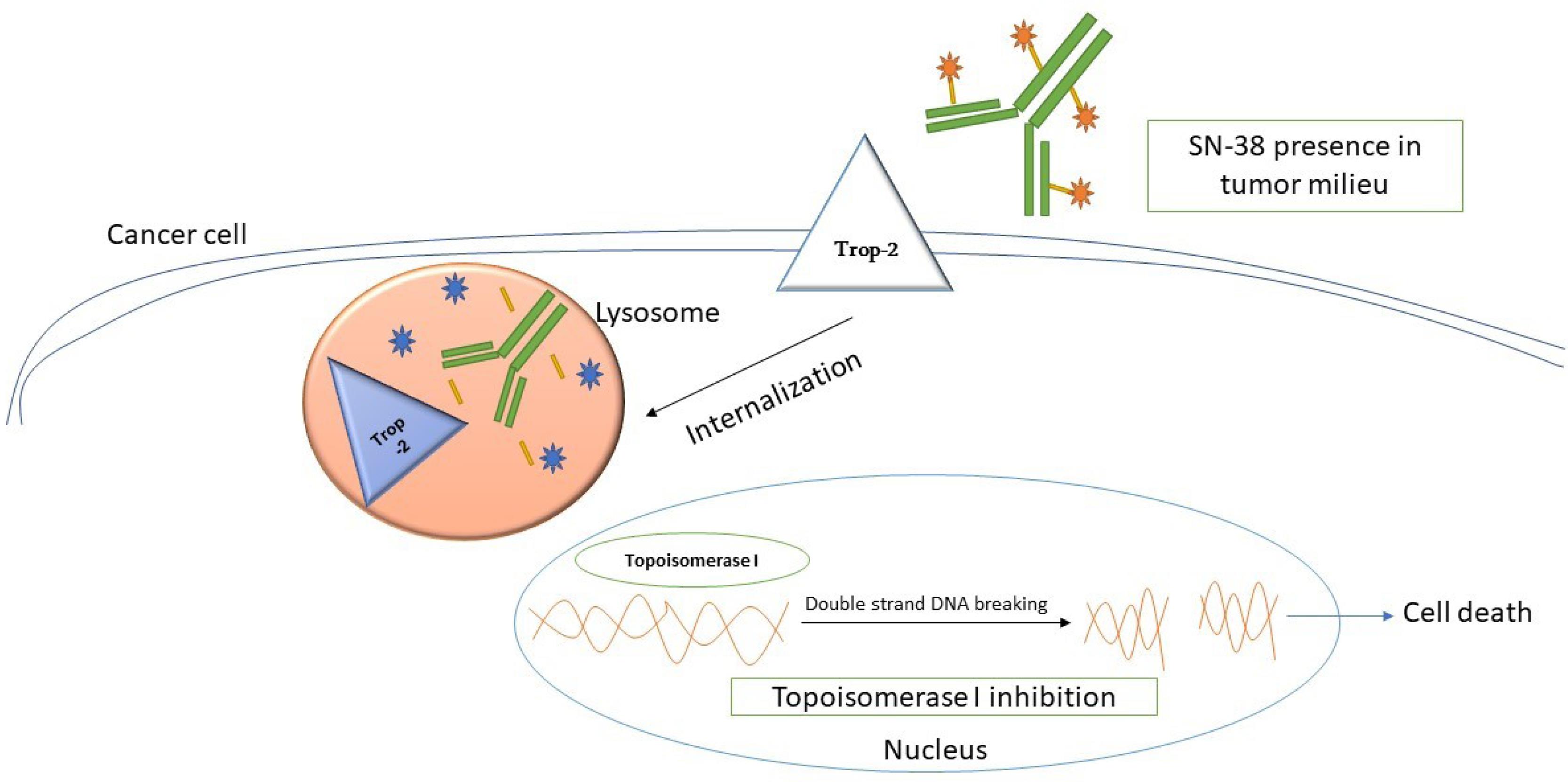
Figure 2.
Mode of Action of Sacituzumab
.
Mode of Action of Sacituzumab
Pharmacokinetics, Pharmacodynamics, and Dosing of Sacituzumab
Sacituzumab has been assessed in the design of Phase I/II basket, multicenter, single-arm clinical trials in individuals whose malignant epithelial tumors had been treated previously.43,44 Based on recent reports of Trop-2 expression in epithelial tumors such as TNBC, monitoring the expression of Trop-2 was deemed unnecessary for determining trial competency.31,34 Twenty-five patients participated in the Phase I trial. Sacituzumab was intravenously injected on days 1 and 8 of a 21-day cycle, and the advised Phase II dose was 11 mg/kg. Neutropenia was the essential dose-confined toxicity, and hopeful anticancer function was reported in individuals with lung, colorectal, and TNBC.35,43 Sacituzumab’s pharmacokinetics, SN-38, IgG, and hRS7 IgG, were evaluated in some patients who participated in the Phase II section and received 9 mg/kg and 11 mg/kg.45,46 During the clinical study, the conjugate received 50% of the SN-38 payload every 24 hours. There were about 11–13 hours of durability for Sacituzumab and 99–108 hours for hRS7 immunoglobulin G (IgG). The area under the curve is about 6 mg-h/mL for Sacituzumab and 14 mg-h/mL for hRs7 IgG. Sacituzumab had a lower distribution volume, 33–37 mL/kg, compared to 57–60 mL/kg for hRs7 IgG. The Sacituzumab clearance rate was about 3 mL/h/kg compared to 0.6 mL/h/kg for hRs7 IgG. SN-38 in serum consists primarily of IgG, which accounts for less than 5% of the total. The SN-38 clearance rate was unaffected by tumor type, and its free serum level was unrelated to neutropenia.
Patients did not develop conjugated antibody responses. SN-38 levels were higher than SN-38G levels due to liver glucuronidation protection by UGT1A1 when RS7 IgG was bound. Over time, the conjugate may release SN-38, resulting in decreased levels of SN-38G in the intestines. Bacterial beta-glucuronidase can then convert it to active SN-38.47. In patients receiving irinotecan, elevated levels of SN-38G may be linked to severe diarrhea.48,49 The in vivo exclusion of Sacituzumab has not been documented in any solemn research. Simplified SN-38 is expected to be removed from Sacituzumab similarly to SN-38 which is expected to be removed from irinotecan.50 During 24 hours of irinotecan injection, only 0.17–0.39% of the drug is recovered in urine as a result of SN-38. Individuals have different biliary exclusions, and SN-38 results in 0.1%–0.9%, while SN-38G results in 0.6%–1.1%. Fecal samples were found to contain SN-38 and a small number of SN-38G concentrations. Initially, SN-38G is excluded from the urine based on glucuronic acid 50’s polar nature. High toxicity with irinotecan is related to ACU1G1 haplotype status, and homozygosity of ACU1G1*28 was assessed in participants in Phase II section.45,51 Regarding statistical data, no significant association was reported between ACU1G1*28 homozygosity and grade more than 3 neutropenia or diarrhea, but these reports showed a slight increase.
Sacituzumab Effects in Metastatic Triple-Negative Breast Cancer
Sacituzumab’s safety and efficacy were assessed in people with progressive TNBC who had previously received at least two medications for malignancy.
Part of Phase I/II Clinical Trial 46
The trial included 110 patients with these conditions who were administered Sacituzumab at a dose of 10 mg/kg intravenously on the first and eighth days of every 21–day cycle. These people were treated with an average of 3 lines of general medication52-57; accordingly, 97% were treated with taxanes and 87% with anthracyclines. The reaction rate to Sacituzumab was 34.6% (95% CI = 23.7–41.8) and included three complete and 29 partial reactions. The average response duration was 8.1 months (95% CI = 5.1–11.2), and the clinical advantage level was 51.3%. The average PFS was 5.2 months (95% CI = 3.9–5.8), and the average total survival was 12.8 months (95% CI = 10.8–14.2).
The quality and efficacy were higher than in previously managed patients with malignant TNBC receiving common treatments after their first line.7,58 Tissue samples from malignant TNBC subtype patients who received Sacituzumab were evaluated for Trop-2 expression.59 Moderate staining was observed in 87% of samples. The PFS of people with mild to significant staining was attempted in some studies, but no significant improvements were observed. The adversary effect profile of Sacituzumab in people who experienced TNBC in metastasis demonstrated similarity to people with other types of cancer.45,46 The highest usual side effects were hematologic and gastrointestinal (Table 1). The most common side effects were neutropenia (59% of all scores, 28% score 3, 15% score 4), nausea (71% of all scores, 5% score 3), and diarrhea (59% of all scores, 7% score 3).33 Although febrile neutropenia is rare, it was reported in 9 patients (8.8%). Deleterious effects, particularly neutropenia, interrupted treatment in 39% of patients. Three patients (3.2%) terminated treatment because of treatment-associated deleterious effects. Sacituzumab has a side effect profile similar to other medications used for TNBC treatment (Table 1).46,60-62 Most patients (93%) received chemotherapy treatments before administration. Particular drugs varied in inpatients, including antihistamines, H2 antagonists, acetaminophen, glucocorticoids, antiemetics, atropine, and anxiolytics.46 Growth factors are commonly utilized to manage treatment-induced neutropenia. It compares Sacituzumab’s efficacy to single-agent medication adoption in a randomized and open-label trial now. Patients with progressive TNBC who have undergone at least two previous treatments are randomly assigned to receive Sacituzumab or the physician’s choice of chemotherapy with eribulin, capecitabine, or gemcitabine in a 1:1 ratio. Independent factors and classification parameters determined the early termination status of the ASCENT trial. These factors include several prior therapy lines, the absence or presence of specified brain metastases, and geographical regions. The second endpoint is OS. Additionally, Sacituzumab showed promising efficacy in treating metastatic TNBC in Phase I/II clinical trials63-66 in HER2-negative hormone receptor-positive BC, urothelial cancer, small cell lung carcinoma, and non-small cell carcinoma.
Table 1.
Most Usual Deleterious Complications Observed in 110 Patients With TNBC (in the Form of Metastasis) Who Received Sacituzumab
46
|
Event
|
All Grades (%)
|
Grade 3–4 (%)
|
| Neutropenia |
59 |
28-15 |
| Nausea |
71 |
5 |
| Fatigue |
57 |
9 |
| Diarrhea |
59 |
7 |
| Infections |
55 |
10 |
| Vomiting |
50 |
8 |
| Anemia |
49 |
9 |
| Constipation |
33 |
2 |
| Alopecia |
40 |
1 |
Note. TNBC: Triple-negative breast cancer.
Conclusion
Sacituzumab is developed as an ADC targeting Trop-2 with suitable function in malignant TNBC, such as other epithelial tumors. Metastasized TNBC is a malignant breast tumor with confined effects from medications other than first-line therapy. Sacituzumab has a controllable adversary effect profile regarding hematologic and gastrointestinal toxicities.
TNBC is defined as cancer without active HER2-directed or hormonal medications. There are no positive adoption parameters that may impact the weak clinical prognosis reported with cytotoxic treatment as a singular treatment for the metastatic option. Atezolizumab in a mixture with nab-paclitaxel for PD-L1.
It is an essential medication for patients with positive TNBC, leading to other immunotherapy combinations for palliative and curative treatments. Active, targeted therapies are essential for 50% of TNBC patients who are negative for PD-L1 and show immunotherapy resistance. Sacituzumab is an excellent ADC due to its unstable connector, which allows for slow secretion of the SN-38 payload over a period of about 49% every 24 hours.The primary form of SN-38 in the blood is IgG, while SN-38G is secreted in the gastrointestinal tract for several days.
It is probable that the antibody will gradually release SN-38, which is the main parameter resulting in Sacituzumab’s efficacy and tolerability in malignant TNBC. Since the success of this approach, the hypothesized future use of this technology is to target other surface markers on various cancer cells. The therapeutic quality of Sacituzumab reported in Phase I/II clinical trials is hopeful compared to the clinical trials of single-element chemotherapy in patients with previous treatments.
Patients with metastatic TNBC demonstrated a poor response (10–15%) to treatment, while Sacituzumab showed a 32.7% response rate 46. The administration of Sacituzumab in these patients, a phase II clinical trial, revealed 5.5 months as the average of PFS. The patients had undergone three rounds of chemotherapy previously, with a range of 2–10.34,63-67 It is better to interpret this cautiously regarding the adoption bias toward healthier patients. Although the ASCENT trial is still ongoing to confirm its effectiveness, we plan to include Sacituzumab in upcoming trials as a neo-adjuvant therapy for patients who did not respond completely to neoadjuvant chemotherapy and are at a high risk of metastatic recurrence. The toxicity profile of Sacituzumab is manageable, and hematologic and gastrointestinal toxicities are the most common high-grade events.46,68,69 Diarrhea rates in TNBC patients were about 8%. This rate is significantly lower than that observed with irinotecan monotherapy, which is reported in 22–29% of patients.47 Four of the 110 patients passed up from the clinical trial because of the deleterious effects associated with Sacituzumab.46 In this trial, most patients were prescribed pre-therapeutics to suppress antiemetics, and the patient’s diarrhea was managed using standard protective measures. Furthermore, growth factors were applied to some patients to treat neutropenic disease. This should be evaluated in future research. The mode of action of Sacituzumab in TNBC was quickly understood due to the updated design of the Phase I/II clinical trial. This design resulted in multiple Phase II clinical trials specific to cancer due to the marked clinical activity observed across various cancer types. According to the primary expression of Trop-2 in epithelial metastatic cancer, Sacituzumab may play a role in treating various types of cancer with TNBC. Sacituzumab is an FDA-approved therapy for metastatic TNBC. This element is used in TNBC in several ways, including concurrent immunotherapy and neoadjuvant therapy for high-risk patients.70 Various medications are being evaluated for treating metastatic BC, including inhibitors of androgen receptors, immunotherapy, phosphatidylinositol-3-kinase/mammalian target of rapamycin pathway suppressors, and PARP suppressors.24,71,72
Acknowledgments
All authors were involved in all stages of the article writing, and they will share all possible material and intellectual rights for this article.
Authors’ Contribution
Conceptualization: Elina Armani Khatibi.
Funding acquisition: Tooba Gholikhani.
Investigation: Balam Jimenez Brito, Nastaran Farshbaf Moghimi.
Project administration:Balam Jimenez Brito, Nastaran Farshbaf Moghimi.
Software: Tooba Gholikhani.
Supervision: Tooba Gholikhani.
Writing–original draft:Elina Armani Khatibi,Balam Jimenez Brito, Nastaran Farshbaf Moghimi.
Writing–review & editing: Tooba Gholikhani.
Competing Interests
All of the authors declare that they have no conflict of interests.
Ethical Approval
Not applicable.
References
- Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet 2016; 293(2):247-69. doi: 10.1007/s00404-015-3859-y [Crossref] [ Google Scholar]
- Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012; 118(22):5463-72. doi: 10.1002/cncr.27581 [Crossref] [ Google Scholar]
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer 2007; 109(9):1721-8. doi: 10.1002/cncr.22618 [Crossref] [ Google Scholar]
- Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol 2010; 33(6):637-45. doi: 10.1097/COC.0b013e3181b8afcf [Crossref] [ Google Scholar]
- Brufsky A, Valero V, Tiangco B, Dakhil S, Brize A, Rugo HS. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat 2012; 133(3):1067-75. doi: 10.1007/s10549-012-2008-6 [Crossref] [ Google Scholar]
- Park IH, Im SA, Jung KH, Sohn JH, Park YH, Lee KS. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED trial (KCSG BR 11-01). Cancer Res Treat 2019; 51(1):43-52. doi: 10.4143/crt.2017.562 [Crossref] [ Google Scholar]
- Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009; 9(1):29-33. doi: 10.3816/CBC.2009.n.005 [Crossref] [ Google Scholar]
- Khosravi-Shahi P, Cabezón-Gutiérrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol 2018; 14(1):32-9. doi: 10.1111/ajco.12748 [Crossref] [ Google Scholar]
- Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer 2018; 96:17-24. doi: 10.1016/j.ejca.2018.03.015 [Crossref] [ Google Scholar]
- Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov 2019; 9(2):176-98. doi: 10.1158/2159-8290.cd-18-1177 [Crossref] [ Google Scholar]
- Lehmann BD, Jovanović B, Chen X, Estrada MV, Johnson KN, Shyr Y. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One 2016; 11(6):e0157368. doi: 10.1371/journal.pone.0157368 [Crossref] [ Google Scholar]
- Metzger Filho O, Stover DG, Asad S, Ansell PJ, Watson M, Loibl S. Association of immunophenotype with pathologic complete response to neoadjuvant chemotherapy for triple-negative breast cancer: a secondary analysis of the BrighTNess phase 3 randomized clinical trial. JAMA Oncol 2021; 7(4):603-8. doi: 10.1001/jamaoncol.2020.7310 [Crossref] [ Google Scholar]
- Echavarria I, López-Tarruella S, Picornell A, García-Saenz J, Jerez Y, Hoadley K. Pathological response in a triple-negative breast cancer cohort treated with neoadjuvant carboplatin and docetaxel according to Lehmann’s refined classification. Clin Cancer Res 2018; 24(8):1845-52. doi: 10.1158/1078-0432.ccr-17-1912 [Crossref] [ Google Scholar]
- Ahn SG, Kim SK, Shepherd JH, Cha YJ, Bae SJ, Kim C. Clinical and genomic assessment of PD-L1 SP142 expression in triple-negative breast cancer. Breast Cancer Res Treat 2021; 188(1):165-78. doi: 10.1007/s10549-021-06193-9 [Crossref] [ Google Scholar]
- Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015; 21(7):1688-98. doi: 10.1158/1078-0432.ccr-14-0432 [Crossref] [ Google Scholar]
- Venetis K, Sajjadi E, Haricharan S, Fusco N. Mismatch repair testing in breast cancer: the path to tumor-specific immuno-oncology biomarkers. Transl Cancer Res 2020; 9(7):4060-4. doi: 10.21037/tcr-20-1852 [Crossref] [ Google Scholar]
- Di Cosimo S. Advancing immunotherapy for early-stage triple-negative breast cancer. Lancet 2020; 396(10257):1046-8. doi: 10.1016/s0140-6736(20)31962-0 [Crossref] [ Google Scholar]
- Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res 2018; 24(3):511-20. doi: 10.1158/1078-0432.ccr-16-3001 [Crossref] [ Google Scholar]
- Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015; 6(7):5449-64. doi: 10.18632/oncotarget.3216 [Crossref] [ Google Scholar]
- Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014; 2(4):361-70. doi: 10.1158/2326-6066.cir-13-0127 [Crossref] [ Google Scholar]
- Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020; 21(1):44-59. doi: 10.1016/s1470-2045(19)30689-8 [Crossref] [ Google Scholar]
- Yoshikawa K, Ishida M, Yanai H, Tsuta K, Sekimoto M, Sugie T. Prognostic significance of PD-L1-positive cancer-associated fibroblasts in patients with triple-negative breast cancer. BMC Cancer 2021; 21(1):239. doi: 10.1186/s12885-021-07970-x [Crossref] [ Google Scholar]
- Dear RF, McGeechan K, Jenkins MC, Barratt A, Tattersall MH, Wilcken N. Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev 2013; 2013(12):CD008792. doi: 10.1002/14651858.CD008792.pub2 [Crossref] [ Google Scholar]
- Wood DE. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Thorac Surg Clin 2015; 25(2):185-97. doi: 10.1016/j.thorsurg.2014.12.003 [Crossref] [ Google Scholar]
- Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 2018; 29(8):1634-57. doi: 10.1093/annonc/mdy192 [Crossref] [ Google Scholar]
- Keung MYT, Wu Y, Vadgama JV. PARP inhibitors as a therapeutic agent for homologous recombination deficiency in breast cancers. J Clin Med 2019; 8(4):435. doi: 10.3390/jcm8040435 [Crossref] [ Google Scholar]
- Torrisi R, Zuradelli M, Agostinetto E, Masci G, Losurdo A, De Sanctis R. Platinum salts in the treatment of BRCA-associated breast cancer: a true targeted chemotherapy?. Crit Rev Oncol Hematol 2019; 135:66-75. doi: 10.1016/j.critrevonc.2019.01.016 [Crossref] [ Google Scholar]
- Keung MY, Wu Y, Badar F, Vadgama JV. Response of breast cancer cells to PARP inhibitors is independent of BRCA status. J Clin Med 2020; 9(4):940. doi: 10.3390/jcm9040940 [Crossref] [ Google Scholar]
- Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, Simeone P. Trop-2 is a determinant of breast cancer survival. PLoS One 2014; 9(5):e96993. doi: 10.1371/journal.pone.0096993 [Crossref] [ Google Scholar]
- Shvartsur A, Bonavida B. Trop-2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer 2015; 6(3-4):84-105. doi: 10.18632/genesandcancer.40 [Crossref] [ Google Scholar]
- Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (Trop-2) as a novel cancer target. Oncotarget 2018; 9(48):28989-9006. doi: 10.18632/oncotarget.25615 [Crossref] [ Google Scholar]
- Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Correction: Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2020; 11(10):942. doi: 10.18632/oncotarget.27512 [Crossref] [ Google Scholar]
- Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015; 6(26):22496-512. doi: 10.18632/oncotarget.4318 [Crossref] [ Google Scholar]
- Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol 2021; 32(6):746-56. doi: 10.1016/j.annonc.2021.03.005 [Crossref] [ Google Scholar]
- Syed YY. Sacituzumab govitecan: first approval. Drugs 2020; 80(10):1019-25. doi: 10.1007/s40265-020-01337-5 [Crossref] [ Google Scholar]
- Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov 2017; 16(5):315-37. doi: 10.1038/nrd.2016.268 [Crossref] [ Google Scholar]
- Adhikari A, Shen B, Rader C. Challenges and opportunities to develop enediyne natural products as payloads for antibody-drug conjugates. Antib Ther 2021; 4(1):1-15. doi: 10.1093/abt/tbab001 [Crossref] [ Google Scholar]
- Shih LB, Xuan H, Aninipot R, Stein R, Goldenberg DM. In vitro and in vivo reactivity of an internalizing antibody, RS7, with human breast cancer. Cancer Res 1995; 55(23 Suppl):5857s-63s. [ Google Scholar]
- Chityala PK, Wu L, Chow DS, Ghose R. Effects of inflammation on irinotecan pharmacokinetics and development of a best-fit PK model. Chem Biol Interact 2020; 316:108933. doi: 10.1016/j.cbi.2019.108933 [Crossref] [ Google Scholar]
- Goldenberg DM, Sharkey RM. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther 2020; 20(8):871-85. doi: 10.1080/14712598.2020.1757067 [Crossref] [ Google Scholar]
- Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res 1991; 51(16):4187-91. [ Google Scholar]
- Sun R, Zhu L, Li L, Song W, Gong X, Qi X. Irinotecan-mediated diarrhea is mainly correlated with intestinal exposure to SN-38: critical role of gut Ugt. Toxicol Appl Pharmacol 2020; 398:115032. doi: 10.1016/j.taap.2020.115032 [Crossref] [ Google Scholar]
- Starodub AN, Ocean AJ, Shah MA, Guarino MJ, Picozzi VJ Jr, Vahdat LT. First-in-human trial of a novel anti-Trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res 2015; 21(17):3870-8. doi: 10.1158/1078-0432.ccr-14-3321 [Crossref] [ Google Scholar]
- Rugo HS, Bardia A, Tolaney SM, Arteaga C, Cortes J, Sohn J. TROPiCS-02: a phase III study investigating sacituzumab govitecan in the treatment of HR + /HER2-metastatic breast cancer. Future Oncol 2020; 16(12):705-15. doi: 10.2217/fon-2020-0163 [Crossref] [ Google Scholar]
- Ocean AJ, Starodub AN, Bardia A, Vahdat LT, Isakoff SJ, Guarino M. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics. Cancer 2017; 123(19):3843-54. doi: 10.1002/cncr.30789 [Crossref] [ Google Scholar]
- Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med 2019; 380(8):741-51. doi: 10.1056/NEJMoa1814213 [Crossref] [ Google Scholar]
- Weiss J, Glode A, Messersmith WA, Diamond J. Sacituzumab govitecan: breakthrough targeted therapy for triple-negative breast cancer. Expert Rev Anticancer Ther 2019; 19(8):673-9. doi: 10.1080/14737140.2019.1654378 [Crossref] [ Google Scholar]
- Xie R, Mathijssen RH, Sparreboom A, Verweij J, Karlsson MO. Clinical pharmacokinetics of irinotecan and its metabolites in relation with diarrhea. Clin Pharmacol Ther 2002; 72(3):265-75. doi: 10.1067/mcp.2002.126741 [Crossref] [ Google Scholar]
- Deyme L, Barbolosi D, Mbatchi LC, Tubiana-Mathieu N, Ychou M, Evrard A. Population pharmacokinetic model of irinotecan and its four main metabolites in patients treated with FOLFIRI or FOLFIRINOX regimen. Cancer Chemother Pharmacol 2021; 88(2):247-58. doi: 10.1007/s00280-021-04255-9 [Crossref] [ Google Scholar]
- Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 2001; 7(8):2182-94. [ Google Scholar]
- Kweekel D, Guchelaar HJ, Gelderblom H. Clinical and pharmacogenetic factors associated with irinotecan toxicity. Cancer Treat Rev 2008; 34(7):656-69. doi: 10.1016/j.ctrv.2008.05.002 [Crossref] [ Google Scholar]
- Asad S, Barcenas CH, Bleicher RJ, Cohen AL, Javid SH, Levine EG. Sociodemographic factors associated with rapid relapse in triple-negative breast cancer: a multi-institution study. J Natl Compr Canc Netw 2021; 19(7):797-804. doi: 10.6004/jnccn.2020.7659 [Crossref] [ Google Scholar]
- Wang X, Wang SS, Huang H, Cai L, Zhao L, Peng RJ. Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA 2021; 325(1):50-8. doi: 10.1001/jama.2020.23370 [Crossref] [ Google Scholar]
- Zhao S, Zuo WJ, Shao ZM, Jiang YZ. Molecular subtypes and precision treatment of triple-negative breast cancer. Ann Transl Med 2020; 8(7):499. doi: 10.21037/atm.2020.03.194 [Crossref] [ Google Scholar]
- Sachdev JC, Munster P, Northfelt DW, Han HS, Ma C, Maxwell F. Phase I study of liposomal irinotecan in patients with metastatic breast cancer: findings from the expansion phase. Breast Cancer Res Treat 2021; 185(3):759-71. doi: 10.1007/s10549-020-05995-7 [Crossref] [ Google Scholar]
- Jain V, Kumar H, Anod HV, Chand P, Gupta NV, Dey S. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J Control Release 2020; 326:628-47. doi: 10.1016/j.jconrel.2020.07.003 [Crossref] [ Google Scholar]
- Garrido-Castro AC, Saura C, Barroso-Sousa R, Guo H, Ciruelos E, Bermejo B. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res 2020; 22(1):120. doi: 10.1186/s13058-020-01354-y [Crossref] [ Google Scholar]
- Chung WP, Yang CT, Chen HY, Su CY, Su HW, Ou HT. Treatment-associated survival outcomes in real-world patients with de novo metastatic triple-negative breast cancer: age as a significant treatment effect-modifier. J Formos Med Assoc 2022; 121(1 Pt 2):319-28. doi: 10.1016/j.jfma.2021.04.028 [Crossref] [ Google Scholar]
- Bardia A, Mayer IA, Diamond JR, Moroose RL, Isakoff SJ, Starodub AN. Efficacy and safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol 2017; 35(19):2141-8. doi: 10.1200/jco.2016.70.8297 [Crossref] [ Google Scholar]
- Petitjean A, Smith-Palmer J, Valentine W, Tehard B, Roze S. Cost-effectiveness of bevacizumab plus paclitaxel versus paclitaxel for the first-line treatment of HER2-negative metastatic breast cancer in specialist oncology centers in France. BMC Cancer 2019; 19(1):140. doi: 10.1186/s12885-019-5335-8 [Crossref] [ Google Scholar]
- Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 2011; 377(9769):914-23. doi: 10.1016/s0140-6736(11)60070-6 [Crossref] [ Google Scholar]
- Feher O, Vodvarka P, Jassem J, Morack G, Advani SH, Khoo KS. First-line gemcitabine versus epirubicin in postmenopausal women aged 60 or older with metastatic breast cancer: a multicenter, randomized, phase III study. Ann Oncol 2005; 16(6):899-908. doi: 10.1093/annonc/mdi181 [Crossref] [ Google Scholar]
- Gray JE, Heist RS, Starodub AN, Camidge DR, Kio EA, Masters GA. Therapy of small cell lung cancer (SCLC) with a topoisomerase-I-inhibiting antibody-drug conjugate (ADC) targeting Trop-2, sacituzumab govitecan. Clin Cancer Res 2017; 23(19):5711-9. doi: 10.1158/1078-0432.ccr-17-0933 [Crossref] [ Google Scholar]
- Heist RS, Guarino MJ, Masters G, Purcell WT, Starodub AN, Horn L. Therapy of advanced non-small-cell lung cancer with an SN-38-anti-Trop-2 drug conjugate, sacituzumab govitecan. J Clin Oncol 2017; 35(24):2790-7. doi: 10.1200/jco.2016.72.1894 [Crossref] [ Google Scholar]
- Tagawa ST, Faltas B, Lam E, Saylor P, Bardia A, Hajdenberg J. Sacituzumab govitecan (IMMU-132) for patients with pretreated metastatic urothelial uancer (UC): interim results. Ann Oncol 2017; 28(Suppl 5):v301-2. doi: 10.1093/annonc/mdx371.012 [Crossref] [ Google Scholar]
- Bardia A, Diamond JR, Vahdat LT, Tolaney SM, O’Shaughnessy J, Moroose RL. Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR + )/HER2-metastatic breast cancer (mBC). J Clin Oncol 2018; 36(15 Suppl):1004. doi: 10.1200/JCO.2018.36.15_suppl.1004 [Crossref] [ Google Scholar]
- Wolber P, Nachtsheim L, Hoffmann F, Klußmann JP, Meyer M, von Eggeling F. Trophoblast cell surface antigen 2 (Trop-2) protein is highly expressed in salivary gland carcinomas and represents a potential therapeutic target. Head Neck Pathol 2021; 15(4):1147-55. doi: 10.1007/s12105-021-01325-5 [Crossref] [ Google Scholar]
- Seligson JM, Patron AM, Berger MJ, Harvey RD, Seligson ND. Sacituzumab govitecan-hziy: an antibody-drug conjugate for the treatment of refractory, metastatic, triple-negative breast cancer. Ann Pharmacother 2021; 55(7):921-31. doi: 10.1177/1060028020966548 [Crossref] [ Google Scholar]
- Spring LM, Nakajima E, Hutchinson J, Viscosi E, Blouin G, Weekes C. Sacituzumab govitecan for metastatic triple-negative breast cancer: clinical overview and management of potential toxicities. Oncologist 2021; 26(10):827-34. doi: 10.1002/onco.13878 [Crossref] [ Google Scholar]
- Cardillo TM, Sharkey RM, Rossi DL, Arrojo R, Mostafa AA, Goldenberg DM. Synthetic lethality exploitation by an anti-Trop-2-SN-38 antibody-drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2-wild-type triple-negative breast cancer. Clin Cancer Res 2017; 23(13):3405-15. doi: 10.1158/1078-0432.ccr-16-2401 [Crossref] [ Google Scholar]
- Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer (Auckl) 2016; 10:25-36. doi: 10.4137/bcbcr.s32783 [Crossref] [ Google Scholar]
- Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O’Shaughnessy J. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol 2018; 36(9):884-90. doi: 10.1200/jco.2016.71.3495 [Crossref] [ Google Scholar]