Biomed Res Bull. 1(2):87-89.
doi: 10.34172/biomedrb.2023.16
Case Report
Coma Following the Sinopharm COVID-19 Vaccine: A Case Report
Nazila Deznabi 1  , Nasrin Abolhasanpour 2, *
, Nasrin Abolhasanpour 2, *  , Hanieh Salehi-Pourmehr 2
, Hanieh Salehi-Pourmehr 2
Author information:
1Shams Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
2Research Center for Evidence-Based Medicine, Iranian EBM Centre: A Joanna Briggs Institute (JBI) Center of Excellence, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
The Chinese Sinopharm vaccine was produced in 2020 as an effective preventive measure against COVID-19. The low and non-serious adverse effects of the Sinopharm COVID-19 vaccine were reported in the literature. This report presents a 70-year-old man who underwent surgical burr-hole evacuation of the hematoma in the right and left parietal and frontal areas. Six days after discharge from the hospital, he received Sinopharm vaccine, and three days post-vaccination, he was referred to the hospital with signs of weakness and lethargy. During the hospitalization, the patient had extensive tonic-clonic seizures and then went into a coma. A close follow-up was done for clinical, laboratory, and imaging studies during his stay in the hospital. After 10 days of appropriate medication, the seizure stopped.
Keywords: Sinopharm, BBIBP-CorV, Hematoma, Convulsions, Coma
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In the last quarter of 2019, in Wuhan in Hubei province, China, many cases of severe lower respiratory tract and lung infections were reported.1,2 In late December, following laboratory investigations, the World Health Organization (WHO) identified the pathogen causing this severe pneumonia as a new strain of coronavirus. The WHO officially named the disease as COVID-19.3,4 In a short time, the virus spread to all world countries and all the populations faced a global health crisis. Accordingly, this situation was declared a pandemic by WHO on March 11, 2020.1,2
Tremendous efforts were taken to rapidly develop and produce COVID-19 vaccines that protect vulnerable individuals from current severe disease and thus limit the health and socioeconomic effects of the pandemic. The Chinese Sinopharm vaccine was produced by Beijing Bio-Institute of Biological Products Company Limited (BBIBP-CorV) as a preventive measure against COVID-19 in 2020.5 According to the updated results (February 25, 2022), 10 vaccines have been granted to emergency use listing by WHO, including Novavax, COVOVAX, Moderna, Pfizer/BioNTech, Janssen (Johnson and Johnson), Oxford/AstraZeneca, Covishield, Bharat Biotech, Sinopharm (Beijing), and Sinovac.6 Minor and non-serious adverse effects were reported in the literature for the Sinopharm COVID-19 vaccine.5 In this study, we report a case who underwent burr-hole drainage of chronic subdural hematoma and showed signs of severe convulsions and coma after receiving the Sinopharm vaccine.
Case Report
The case was a 70-year-old man diagnosed and hospitalized with a chronic subdural hematoma on computed tomography (CT) scan image. He had been referred to the hospital complaining of weakness in the upper and lower left limbs, imbalance, and inability to walk. The patient’s medical history showed diabetes mellitus and hypertension, both of which were controlled with appropriate medications.
One month before hospitalization, the patient had fallen and suffered a head injury.
One day after hospitalization, he underwent surgical burr-hole evacuation of the hematoma in three areas: frontal, right, and left parietal. He was transferred to the surgical intensive care unit postoperatively while he was awake and hemodynamically stable. The next day, he was transferred to the ward, and he was discharged from hospital two days later with instructions to rest at home with no heavy work.
He had received the Sinopharm vaccine six days after discharge from the hospital. Three days postvaccination, he was referred to the hospital complaining of weakness, lethargy, and dysuria. CT scan and brain magnetic resonance imaging (MRI) did not show any hematoma or pressure on the brain (Figure 1). Additionally, no evidence in favor of convulsion was found. The next day, antibiotics were started. We performed tests for the diagnosis of meningitis on patients. Extensive tonic-clonic seizures were observed, followed by a short coma. Phenytoin (800 mL/kg within 20 minutes) and Diazepam (100 mL/kg every 8 hours) were administered. Despite receiving diazepam, the seizures did not stop. We observed decreased consciousness despite the above-mentioned interventions. Due to the patient’s unconsciousness, Levetiracetam (500 mL/kg every 2 hours) was infused on the sixth day of the hospitalization. However, the seizures continued and they were difficult to control. Ten days after hospitalization, the seizure stopped.
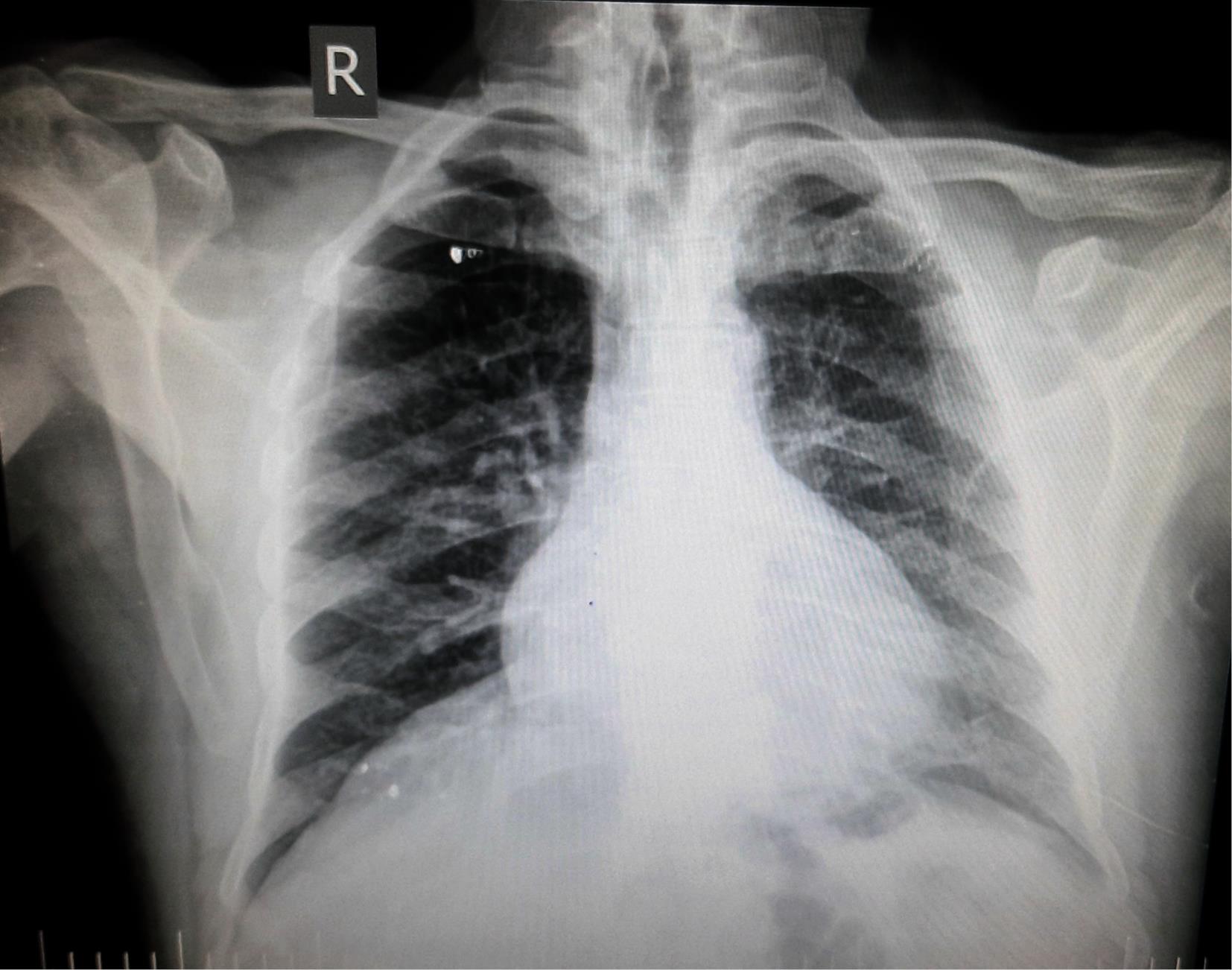
Figure 1.
Chest CT Scan of the Patient
.
Chest CT Scan of the Patient
Discussion
The proposed neuropathogenesis of SARS-CoV-2 has not been established completely. However, the most accepted theories include immune complex-mediated reactions, retrograde axonal transmission, angiotensin-converting enzyme-2 (ACE-2) associated virus invasion, infected leukocyte-mediated transportation (Trojan horse), endothelial impairment in the blood–neuron interface, thrombotic microangiopathy, and cytokine storm.7-9
The pathophysiology related to nervous system manifestations of COVID-19 vaccines has not been identified yet. It is most relevant to common pathways involved in immunologic responses of vaccines and virus. The degree of virus inactivation-reactivation, systemic response related to cytokines, immune complex-mediated reactions, and complications associated with vector are suggested.10,11
Despite the efforts to develop a vaccine against COVID-19, the side effects of vaccines remain an issue of concern among the public. El-Shitany et al12 showed that most of the adverse effects of vaccines occurred after the second dose. According to the FDA fact sheet for recipients and caregivers, the most common adverse reactions of the Pfizer COVID-19 vaccine include headaches, pain at the injection site, increase in body temperature, tiredness, muscle and joint pain, and chills. These signs could last for several days and were mostly observed after the second shot. Rarely reported side effects include Bell’s palsy, lymph node tenderness, and swelling 12. Based on the WHO announcement, the Sinopharm COVID-19 vaccine showed mild to a moderate side effect in phase 3 clinical trials on 16671 participants aged 18–59 years, such as injection site reactions, headache, and fatigue, which were the most common complications.13 In the study conducted by Al Khames et al,14 it was found that 40% of those who received Sinopharm did not report any signs and symptoms. This rate was 25.71% for Pfizer vaccine and 18.39% for AstraZeneca vaccine. Moreover, it was reported that the side effects of the AstraZeneca vaccine were more prevalent after the first dose, followed by Pfizer, and those who received the Sinopharm vaccine showed fewer adverse reactions. In most vaccinated individuals, weakness and numbness in the injected arm were the most commonly reported side effects.14,15 Longer post-vaccination symptoms and higher risk of signs were observed in AstraZeneca vaccine compared to Pfizer and Sinopharm vaccines.15 A case study reported an 82-year-old patient with dementia. Following vaccination with the mRNA-based Pfizer–BioNTech COVID-19 vaccine, the patient had a reactivation of hepatitis C virus infection, which manifested with jaundice, loss of consciousness, hepatic coma, and death.16 Another study reported a 67-year-old female patient who was hospitalized because of right-sided hemiparesis and pain in the neck with rapid deterioration to a deep coma 2 days after receiving the Sinopharm vaccine. She was discharged from the hospital after receiving two months of intensive medical care.11 Ancau et al17 reported a 61-year-old male patient with a history of polymyalgia rheumatica and hypothyroidism who developed headache, fever, and apathy two days after the first shot of the ChAdOx1 nCoV-19 (AZD1222 [Covishield]; AstraZeneca) vaccine. Two days after receiving the vaccine, his wife found him unconscious in his bed foaming around the mouth. He had a generalized seizure, was subsequently comatose, and underwent endotracheal intubation. Clinical rehabilitation follow-up lasted for 14 weeks, and the patient presented with a vegetative state.
In different studies, controversial findings were reported on the safety, adverse reaction, and neuropathogenesis of COVID-19 vaccines, which demand more investigations and reports.
Conclusion
Overall, both at population and individual levels, the benefits of COVID-19 vaccination far outweigh the risks of neurological complications. However, Sinopharm vaccine, despite mild reported post-vaccination signs and symptoms, should be injected warily in patients who underwent surgical burr-hole evacuation of the subdural hematoma.
Acknowledgments
We express our appreciation to the staff of the Department of Neuroscience, Shams Hospital, Tabriz University of Medical Sciences, Tabriz, Iran.
Authors’ Contribution
Conceptualization: Nazila Deznabi.
Data curation: Nasrin Abolhasanpour.
Formal analysis: Hanieh Salehi-Pourmehr.
Funding acquisition: Hanieh Salehi-Pourmehr.
Investigation: Nazila Deznabi.
Methodology: Nazila Deznabi.
Project administration: Nasrin Abolhasanpour.
Resources: Nazila Deznabi.
Software: Nasrin Abolhasanpour.
Supervision: Hanieh Salehi-Pourmehr.
Validation: Hanieh Salehi-Pourmehr.
Visualization: Hanieh Salehi-Pourmehr.
Writing–original raft: Nazila Deznabi.
Competing Interests
The authors declare no conflict of interests.
Consent for Publication
Informed consent was obtained from the patient for publication of this case report.
Funding
No financial support was received for this work.
References
- Mostafaei A, Ghojazadeh M, Hajebrahimi S, Abolhasanpour N, Salehi-Pourmehr H. Clinical presentation of Iranian patients affected with COVID-19: a thousand faces disease. Iran J Allergy Asthma Immunol 2021; 20(2):140-6. [ Google Scholar]
- Mostafaei A, Hajebrahimi S, Sadeghi-Ghyassi F, Mostafaei H, Abolhasanpour N, Nasseri A. Can wearing a face mask protect from COVID-19? A systematic review. Iran J Med Microbiol 2020; 14(2):101-7. doi: 10.30699/ijmm.14.2.101 [Crossref] [ Google Scholar]
- Shahsavarinia K, Faridaalaee G, Soleimanpour H, Sadeghi-Ghyassi F, Atashgahi S, Milanchian N. Cerebral venous thrombosis (CVT) following COVID-19 vaccination: an umbrella review of systematic reviews. Iran J Med Microbiol 2023; 17(1):7-21. doi: 10.30699/ijmm.17.1.7 [Crossref] [ Google Scholar]
- Salehi-Pourmehr H, Dolati S, Mehdipour R, Memar A, Ghafourian F, Shakiba A. Effect of montelukast on treatment of coronavirus pneumonia (COVID-19): a systematic review. Biomed Res Bull 2023; 1(1):19-29. doi: 10.34172/biomedrb.2023.06 [Crossref] [ Google Scholar]
- Abu-Riash A, Tareef AB, Aldwairy A. Could Sinopharm COVID-19 vaccine cause autoimmune encephalitis? A case report. J MAR Neurol 2021; 3(5):1-5. [ Google Scholar]
- World Health Organization (WHO). 10 Vaccines Granted Emergency Use Listing (EUL) by WHO. Available from: https://covid19.trackvaccines.org/agency/who/. February 25, 2022. Accessed February 28, 2022.
- Leven Y, Bösel J. Neurological manifestations of COVID-19 - an approach to categories of pathology. Neurol Res Pract 2021; 3(1):39. doi: 10.1186/s42466-021-00138-9 [Crossref] [ Google Scholar]
- Vitalakumar Vitalakumar, D D, Sharma A, Kumar A, Flora SJS. Neurological manifestations in COVID-19 patients: a meta-analysis. ACS Chem Neurosci 2021; 12(15):2776-97. doi: 10.1021/acschemneuro.1c00353 [Crossref] [ Google Scholar]
- Collantes MEV, Espiritu AI, Sy MCC, Anlacan VMM, Jamora RDG. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci 2021; 48(1):66-76. doi: 10.1017/cjn.2020.146 [Crossref] [ Google Scholar]
- Lu L, Xiong W, Mu J, Zhang Q, Zhang H, Zou L. The potential neurological effect of the COVID-19 vaccines: a review. Acta Neurol Scand 2021; 144(1):3-12. doi: 10.1111/ane.13417 [Crossref] [ Google Scholar]
- Sourani A, Rezvani M, Foroughi M, Baradaran Mahdavi S. Spontaneous intramedullary hematoma following COVID-19 vaccination: a case report. Clin Case Rep 2022; 10(12):e6743. doi: 10.1002/ccr3.6743 [Crossref] [ Google Scholar]
- El-Shitany NA, Harakeh S, Badr-Eldin SM, Bagher AM, Eid B, Almukadi H. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med 2021; 14:1389-401. doi: 10.2147/ijgm.s310497 [Crossref] [ Google Scholar]
- Saeed BQ, Al-Shahrabi R, Alhaj SS, Alkokhardi ZM, Adrees AO. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int J Infect Dis 2021; 111:219-26. doi: 10.1016/j.ijid.2021.08.013 [Crossref] [ Google Scholar]
- Al Khames Aga QA, Alkhaffaf WH, Hatem TH, Nassir KF, Batineh Y, Dahham AT. Safety of COVID-19 vaccines. J Med Virol 2021; 93(12):6588-94. doi: 10.1002/jmv.27214 [Crossref] [ Google Scholar]
- Matarneh AS, Al-Battah AH, Farooqui K, Ghamoodi M, Alhatou M. COVID-19 vaccine causing Guillain-Barre syndrome, a rare potential side effect. Clin Case Rep 2021; 9(9):e04756. doi: 10.1002/ccr3.4756 [Crossref] [ Google Scholar]
- Lensen R, Netea MG, Rosendaal FR. Hepatitis C virus reactivation following COVID-19 vaccination - a case report. Int Med Case Rep J 2021; 14:573-6. doi: 10.2147/imcrj.s328482 [Crossref] [ Google Scholar]
- Ancau M, Liesche-Starnecker F, Niederschweiberer J, Krieg SM, Zimmer C, Lingg C. Case series: acute hemorrhagic encephalomyelitis after SARS-CoV-2 vaccination. Front Neurol 2021; 12:820049. doi: 10.3389/fneur.2021.820049 [Crossref] [ Google Scholar]