Biomed Res Bull. 1(2):42-47.
doi: 10.34172/biomedrb.2023.09
Original Article
Silymarin Potentiates the Effect of Buspirone on Anxiety and Depressive-Like Behaviors Induced by Chronic Restraint Stress in Male Mice
Sevil Rajabi 1, 2  , Alireza Mohajjel Nayebi 2, 3, *
, Alireza Mohajjel Nayebi 2, 3, *  , Aytak Khabbaz 1, Naeimeh Hosseinzadeh 1, Leila Hosseini 1, Shadi Mohajjel Naebi 1
, Aytak Khabbaz 1, Naeimeh Hosseinzadeh 1, Leila Hosseini 1, Shadi Mohajjel Naebi 1
Author information:
1Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Pharmacology and Toxicology, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Stress and its complications such as anxiety and depression continue to be regarded as health issues in human societies. Therefore, an effective treatment strategy is highly valued for dealing with stress and related disorders. In spite of studies suggesting the neuroprotective effects of silymarin and buspirone alone, the combined effects of these treatments on chronic stress have not been elucidated yet. Thus, this study aimed to investigate the effect of silymarin and buspirone (alone or in combination) on anxiety and depressive-like behaviors and to evaluate corticosterone levels in a mice model of chronic restraint stress (RS).
Methods:
This study was conducted on seventy-two male BALB/c mice which were allocated in six equal groups. The animals were exposed to chronic RS (2 hours/day for 14 days) to induce a depressive-like model. An elevated plus maze (EPM) was performed to assess anxiety, while a tail suspension test (TST) was implemented to evaluate depressive-like behavior. Furthermore, the serum levels of corticosterone were measured by the enzyme-linked immunosorbent assay method.
Results:
Our data demonstrated that exposure to RS resulted in prolonged immobility in the TST and reduced time spent in the open arms of the EPM test. Buspirone perorally (5 mg/kg, PO) alone and combined with silymarin (200 mg/kg, PO) increased time spent in the open arms of the EPM apparatus while attenuating immobility time in TST. There was a significant decrease in the blood corticosterone level of buspirone and silymarin co-treated animals.
Conclusion:
These findings indicated that silymarin potentiates the beneficial effect of buspirone against chronic RS-induced anxiety and depressive-like behaviors in mice by lowering corticosterone serum levels. In this regard, further investigations should be undertaken to clarify the exact mechanism of the observed effects.
Keywords: Silymarin, Buspirone, Anxiety, Depression, Restraint stress
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Stress as a negative stimulus affects homeostasis, the natural regulation of living organisms, physiological responses, and adaptation.1 Nowadays, modernization, industrialization, and changes in human lifestyle have created a particular situation that is the source of various stressors.2 However, enduring stressors may produce chronic stress, which leads to changes in brain structure.3 In addition, it reduces the volume of various brain areas, including the hippocampus, amygdala, and frontal cortex (areas with the highest glucocorticoid [GC] receptors and represent the most elevated stress response), which can cause reversible cognition and spatial memory impairment.4 It has been demonstrated that prolonged exposure to stressors causes the activation of the hypothalamus-hypophyseal-adrenal (HPA) axis, a neuroendocrine pathway consisting of the hypothalamus, pituitary, and adrenal glands.1 The end-product of the HPA axis is a GC secreting from adrenal glands.5 GC has a prominent role in stress-related homeostasis, behavior, and cognition.6,7 Further, elevated GC levels increase the production of free radicals and reactive oxygen species.8,9
Clinical and experimental research has demonstrated that stress is associated with increased psychiatric disorders such as depression and anxiety.10-12 The most debilitating complication of stress is depression, which affects the body’s overall function, including the cardiovascular,13,14 gastrointestinal,15 immune,16 and brain structure and function.9,17 In addition, depression is a profound public health concern with various physical ailments, reduced social functioning, a significant cause of disability, and increased mortality. It imposes a heavy burden on communities.18,19
Benzodiazepine drugs have been a choice therapy for anxiety symptoms for several decades. These drugs have well-known side effects such as drowsiness, lethargy, cognitive and motor impairment, and depression in some patients despite the effective anxiolytic impact. Hence, benzodiazepines can be replaced by nonbenzodiazepine drugs such as buspirone without nervous system side effects.20,21 Buspirone is a partial agonist of the 5-HT1A receptor prescribed to treat patients with generalized anxiety disorders and common anxiety behaviors in adults and generally in people who experience anxiety and depression at different ages.22
Mary thistle (Silybum marianum L. Gaernt.) is a medicinal herb that has been used for hepatoprotective, anti-inflammatory, and antioxidant properties.23 Recent research has investigated the potential of silymarin in the central nervous system disorders such as Alzheimer’s disease, Parkinson’s disease, anxiety, and the treatment of depression by influence on catecholamine neurotransmitter systems such as serotonin, dopamine, and noradrenergic.24-29 Silymarin extract can prevent apoptosis due to oxidative stress and delayed neuronal death in hippocampal neurons.24,30 Recent studies on different types of stress illustrated that silymarin has beneficial effects on cognitive deficits, anxiety, and depression in long-term treatment.31,32 Numerous studies revealed that chronic restraint stress (RS) is one of the standard ways to induce depressive-like behaviors, the consequences of chronic stress in rodents such as mice.2,33 Therefore, the current study was conducted to investigate whether a combination of silymarin with buspirone has potentiating effects against anxiety and depression-like behaviors and regulation of corticosterone levels in RS-subjected mice.
Materials and Methods
Animals
Male BALB/c mice (8-10 weeks old, 28-32 g body weight) were obtained from the animal center of Tabriz University of Medical Sciences. The animals were housed in a temperature-controlled room, with 60%-65% relative humidity, a 12/12-hour dark/light cycle, and had free access to rodent diet and tap water.
Drugs Administration
After a week of environmental adaptation, the mice were randomly divided into six experimental groups of 12, including a control group that received normal saline (NS) perorally (PO) for 14 days. The other five groups underwent RS and subsequently received 14 days of treatment PO as RS + NS (10 mL/kg), RS + Bus (5 mg/kg, buspirone), RS + Sil (100 mg/kg, silymarin), RS + Sil (200 mg/kg), and RS + Bus (5 mg/kg, buspirone) + Sil (200 mg/kg). All drug solutions were prepared freshly on the day of experimentation by dissolving in NS. The determination of buspirone and silymarin dose in this study was based on previous studies, indicating that they have beneficial effects on reducing stress complications using other induction models.24,34,35
Chronic Restraint Stress
The animals were exposed to chronic RS by being placed in 50 mL-plastic tubes with 12 holes to keep airflow for two hours (10-12 am) once daily for 14 consecutive days. Control mice were kept in their home cages in the animal room, except for the daily handling and NS administration.
Elevated Plus Maze
The anxiety-like behaviors were measured using the elevated plus maze (EPM) test. The maze consisted of four arms (10 cm wide and 50 cm long); two opened opposite arms with 1 cm walls and two closed opposite arms are surrounded by a transverse wall 40 cm in height. These four arms form a square intersection (10 cm wide and 10 cm long), and the apparatus is elevated to a height of 55 cm above the floor. A mouse was placed at the intersection, facing the closed arm, and moved freely in different parts of the maze for five minutes during the experiment. The number of open arms entries and the time spent in the open arms were calculated as follows:
Percentage of time spent in the opened arm = Time spent in the open arm / Total time spent in open and closed arms *100
Percentage of open arm entries = The number of times entered the open arm / Total number of times entered the open and closed arms *100
The presence of a significant increase in these two parameters indicates that anxiety was lower in the mice.36
Tail Suspension Test
Each mouse was suspended 50 cm above the floor using adhesive tape for 6 minutes by the tail (2 cm from the end of the tail). The immobility time was calculated at the last 4 minutes. Immobility time was defined as the lack of escape-oriented behavior.37
Behavioral Analysis
All behavioral data were analyzed using Noldus EthoVisionTM video tracking software (Noldus, The Netherlands). After testing each mouse, the device was cleaned with 10% ethanol to remove residues and olfactory clues.
Sampling and Corticosterone Evaluation
For the biochemical test, mice were anesthetized with high doses of ketamine (90 mg/kg) and xylazine (10 mg/kg) between 10 and 12 AM. 24 hours after the end of the behavioral test. The blood samples were taken from the heart and centrifuged at 1500 × g for 10 minutes at 4 °C. According to the manufacturer’s instructions, serum corticosterone levels were measured by a commercially specific enzyme-linked immunosorbent assay kit (Abnova Corporation, Walnut, CA, USA).38
Statistical Analysis
All data are expressed as the mean ± standard error of the mean (SEM). Statistical differences between study groups were analyzed using the analysis of variance and Tukey statistical methods and GraphPad Prism 6.01 software. Additionally, P values < 0.05 were considered statistically significant.
Results
Effects of Co-administration of Silymarin and Buspirone on Anxiety and Depressive Behaviors
The results of the EPM test revealed that there was a significant difference in the percentage of entry (open arms entries [OAE]) and the percentage of spent time (open arms times [OAT]) in the open arms between the mice of RS and NS groups (P < 0.001, Figures 1A and 1B, respectively). However, treatment with buspirone (5 mg/kg, PO, P < 0.05) and co-administration of buspirone (5 mg/kg, PO) with silymarin (200 mg/kg, PO, P < 0.01) represented a significant increase in the percentage of OAE and OAT in the restraint-exposed mice.
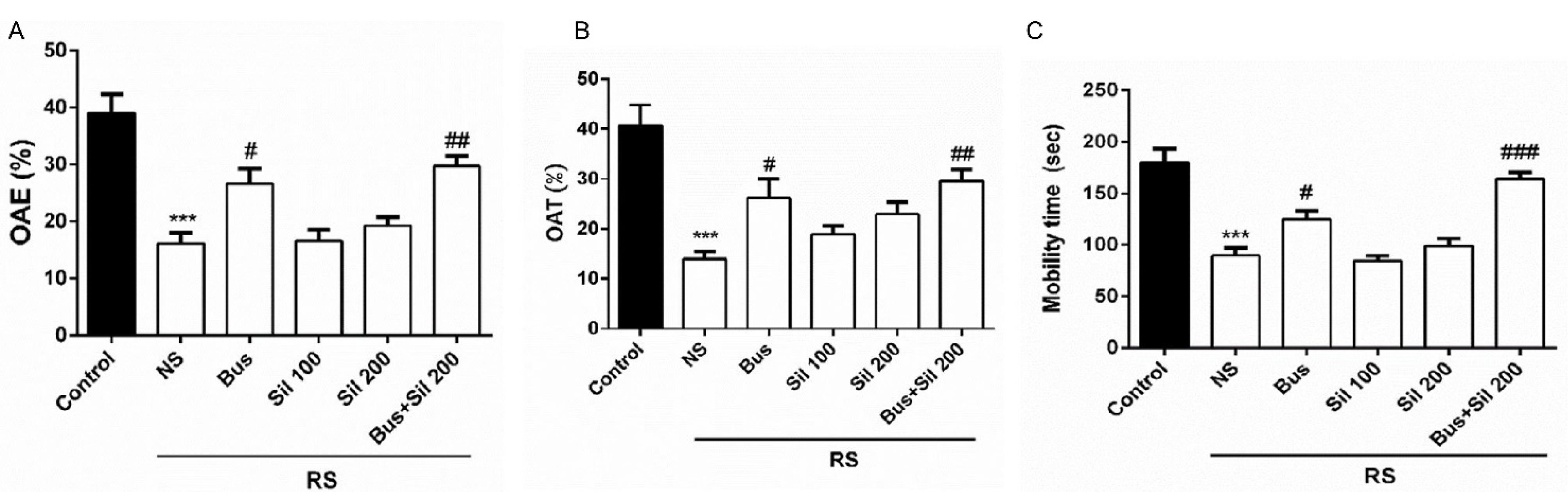
Figure 1.
Effects of Co-administration of Buspirone and Silymarin on (A) the %OAE, (B) %OAT in the EPM, and (C) Immobility Time in TST. Note. Data are presented as the mean ± SEM (n = 12 in each group). ***P < 0.001 in comparison with control group; #P < 0.05, ##P < 0.01, and ###P < 0.001 in comparison with the NS group. SEM: Standard error of the mean; Bus: Buspirone, Sil: Silymarin, %OAT: Time spent in open arms; %OAE: Open arm entries, EPM: Elevated plus, TST: Tail suspension test, NS: Normal saline
.
Effects of Co-administration of Buspirone and Silymarin on (A) the %OAE, (B) %OAT in the EPM, and (C) Immobility Time in TST. Note. Data are presented as the mean ± SEM (n = 12 in each group). ***P < 0.001 in comparison with control group; #P < 0.05, ##P < 0.01, and ###P < 0.001 in comparison with the NS group. SEM: Standard error of the mean; Bus: Buspirone, Sil: Silymarin, %OAT: Time spent in open arms; %OAE: Open arm entries, EPM: Elevated plus, TST: Tail suspension test, NS: Normal saline
The post hoc test showed that chronic RS significantly reduced mobility time in the tail suspension test (TST) between the control and NS groups (P < 0.001, Figure 1C). In contrast, in buspirone-treated mice, mobility time significantly increased compared to the RS group (P < 0.05). Furthermore, co-administration of silymarin (200 mg/kg, PO) with buspirone (5 mg/kg, PO) had an antidepressant-like activity, which was manifested by the shortening of the duration of immobility (P < 0.001).
The Effect of Co-administration of Silymarin and Buspirone on Serum Corticosterone Levels
As shown in Figure 2, the serum corticosterone level was significantly increased in the RS group compared to the control group (P < 0.001). Co-administration of silymarin (200 mg/kg, PO) as the effective dose with buspirone (5 mg/kg, PO) markedly decreased the corticosterone level when compared with the RS + NS group (P < 0.001). Furthermore, the corticosterone levels were significantly decreased in buspirone (5 mg/kg, PO) and RS-exposed mice (P < 0.01).
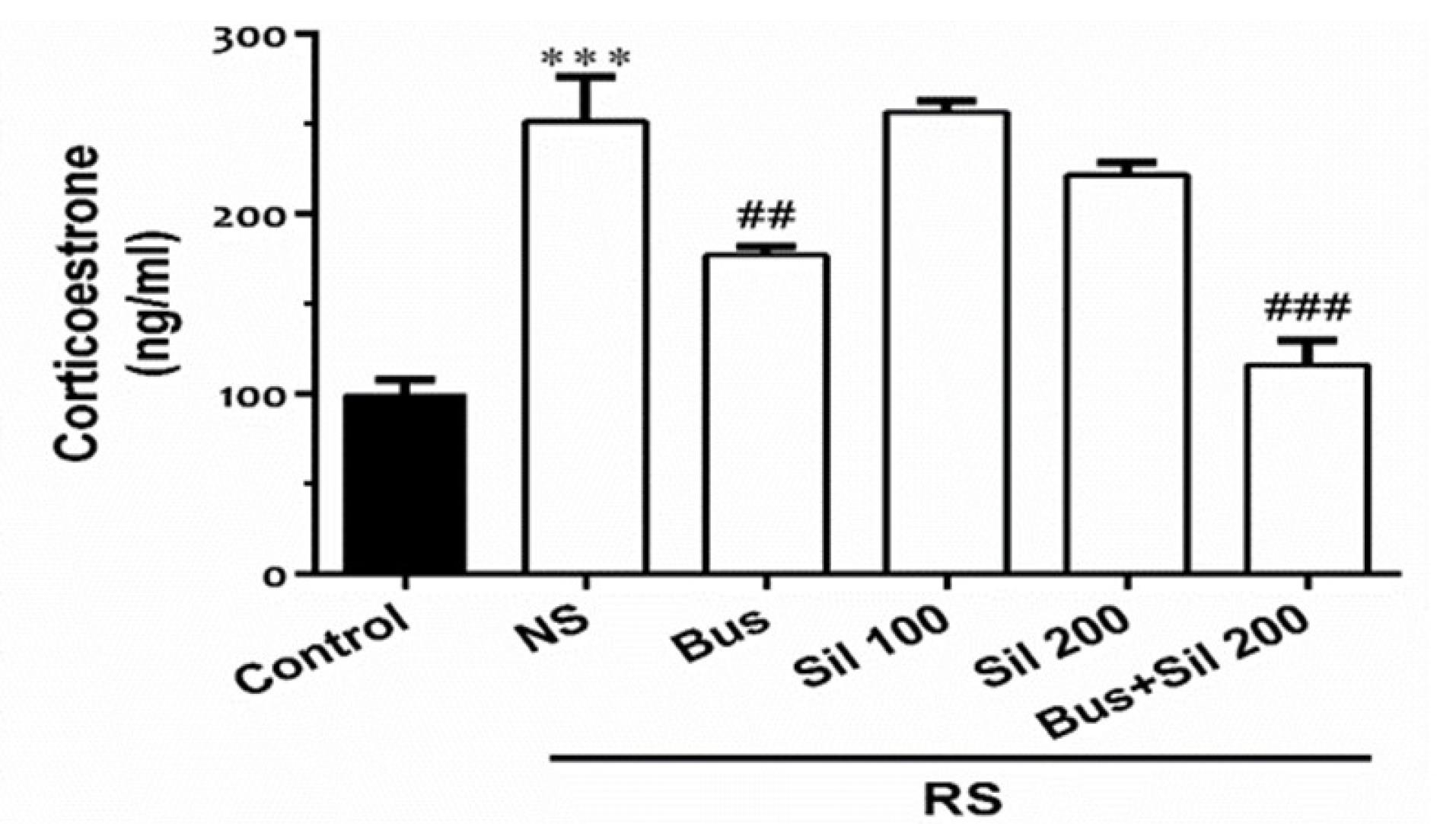
Figure 2.
The Effect of Co-administration of Silymarin and Buspirone on Serum Corticosterone Levels. Note. Data are presented as the mean ± SEM (n = 12 in each group). ***P < 0.001in comparison with the control group; ##P < 0.01 and ###P < 0.001 in comparison with the NS + RS group. Bus: Buspirone, Sil: Silymarin, RS: Restraint Stress, NS: Normal Saline
.
The Effect of Co-administration of Silymarin and Buspirone on Serum Corticosterone Levels. Note. Data are presented as the mean ± SEM (n = 12 in each group). ***P < 0.001in comparison with the control group; ##P < 0.01 and ###P < 0.001 in comparison with the NS + RS group. Bus: Buspirone, Sil: Silymarin, RS: Restraint Stress, NS: Normal Saline
Discussion
There is growing evidence indicating an increase in combination drugs used to treat psychiatric and physical symptoms and diseases. On the other hand, herbal remedies are traditionally employed to treat diseases experimentally without having scientific information about their effects and side effects on patients.39,40
The present study investigated the effect of two doses of silymarin (200 and 100 mg/kg) and co-administration with buspirone (5 mg/kg) on anxiety and depression behaviors due to chronic RS in male mice. The results demonstrated that treatment with buspirone for 14 days in chronic RS mice improved anxiety and depressive-like behaviors by the increasing number of entries and spending more extended time in open arms of the EPM apparatus. Moreover, mobility time in the TST task was longer than in the RS group. The combination of buspirone with silymarin (200 mg/kg) more potentiated their protective effect, suggesting the therapeutic potential against RS-induced, anxiety, and depression-like behaviors. In addition, serum corticosterone levels remarkably decreased in combination treatment and buspirone alone in RS-subjected mice. These findings indicated the anxiolytic and anti-depressant effects of buspirone and a combination of silymarin and buspirone. However, the co-administration of these two drugs was more effective than each drug alone.
As one of the standards and accepted methods of stress induction in rodents, RS is a widely applied stress model. This method can be utilized inexpensively and quickly and does not harm the animal physically.41 It also causes biochemical, neurophysiological, and behavioral changes in laboratory animals, and the results in the clinic can be generalized to human cases.42 Furthermore, the findings revealed that exposure to acute or chronic RS causes anxiety and depression in rodents.43 In the current study, movement restriction due to RS (for 14 consecutive days and 2 hours per day) could lead to anxiety and depression. This finding is in line with those of some studies, indicating that inducing RS not only causes depressive and anxiety behaviors but can also be associated with complications such as cognitive impairment and appetite.42,44
Buspirone is administrated to treat anxiety and depression-like symptoms by the partial serotonin receptor agonist mechanism. It affects serotonin receptors located in the CA1 region of the hippocampus and cerebral cortex.22 Animal models have shown that low doses (1 mg/kg) of this drug activate pre-synaptic autoreceptors, and high doses (2.0 mg/kg) stimulate post-synaptic receptors, thus the effects of this drug depend on the dose. It has also been indicated that high doses increase locomotion.45,46 Some studies demonstrated that the imbalance of the serotonergic system increases anxiety; accordingly, for the treatment of anxiety symptoms, anxiolytic drugs have been used that act on the serotonin system by inhibiting serotonin reuptake and serotonin 1A receptor agonist (5-HT1AR).32,47 Studies have documented that mice exposed to RS increased their fear. Hence, NS mice treated with serotonergic drugs moved more time in the open arm, and mobility time increased on the TST, whereas it was not observed in the NS pretreatment group.48
Several lines of studies in animal models have proven that silymarin (100 and 200 mg/kg) has antidepressant and neuroprotection activity. Moreover, it can pass through blood-brain barrier and affect serotonin (5-HT), dopamine, and norepinephrine receptors in the brain, increase the level of these hormones in the blood, and lessen behavioral and biochemical in ischemic rat models.32,49 Thakare et al, in an in vivo study on acute restrained stress mice, demonstrated that after treatment with silymarin 100 and 200 mg/kg, mobility time in forced swimming test increased, and it had an antidepressant effect.35
The HPA axis is activated as one of the most critical neuroendocrine systems in response to stressors, leading to the release of GCs from the adrenal glands, including cortisol in humans and corticosterone in rodents.50 Disturbances in this axis can cause disruptions in synaptic plasticity and lead to depression by exacerbating neuronal damage.51 Previous studies have pointed out that stressful events, especially chronic RS, can cause the dysregulation of this axis by disrupting the harmful feedback activity. Hence, it leads to the overstimulation of the axis and causes an unusual increase in serum cortisol and corticosterone in humans and rodents, respectively, leading to brain damage.52,53 The three regions of the brain such as the hippocampus, frontal cortex, and amygdala are susceptible to long-term exposure to stress, causing increased GC expression in these structures and leading to physiological changes and disorders.54,55 In line with previous studies,32,35,56 our result represented that exposure to chronic RS increased serum corticosterone levels in mice compared to the control group. Moreover, co-administration of buspirone and silymarin (200 mg/kg) and buspirone alone in RS-subjected mice significantly decreased corticosterone levels in the serum.
Conclusion
In general, the present findings indicated that the co-administration of silymarin and buspirone exerts a potentiating effect against biochemical and behavioral alterations induced by chronic RS stress. Nevertheless, more molecular and behavioral studies should be performed to reveal their exact antianxiety and antidepressant mechanisms.
Acknowledgments
The authors would like to thank the Dean of the Faculty of Pharmacy and Drug Applied Research Center of Tabriz University of Medical Sciences (Tabriz, Iran) for supporting this study.
Authors’ Contribution
Data curation: Alireza Mohajjel Nayebi.
Formal analysis: Sevil Rajabi.
Funding acquisition: Alireza Mohajjel Nayebi, Aytak Khabbaz.
Investigation: Naeimeh Hosseinzadeh.
Methodology: Leila Hosseini.
Project administration: Alireza Mohajjel Nayebi.
Resources: Shadi Mohajjel Naebi.
Software: Alireza Mohajjel Nayebi.
Supervision: Alireza Mohajjel Nayebi.
Validation: Aytak Khabbaz.
Visualization: Naeimeh Hosseinzadeh.
Writing–original draft: Leila Hosseini, Aytak Khabbaz.
Competing Interests
The authors have no conflict of interests to disclose.
Ethical Approval
All animal experiments were performed in accordance with the guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996) and were approved by the Ethical Committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1397.318).
References
- Vyas S, Rodrigues AJ, Silva JM, Tronche F, Almeida OF, Sousa N. Chronic stress and glucocorticoids: from neuronal plasticity to neurodegeneration. Neural Plast 2016; 2016:6391686. doi: 10.1155/2016/6391686 [Crossref] [ Google Scholar]
- Becerril-Chávez H, Colín-González AL, Villeda-Hernández J, Galván-Arzate S, Chavarría A, Eduarda de Lima M. Protective effects of S-allyl cysteine on behavioral, morphological and biochemical alterations in rats subjected to chronic restraint stress: antioxidant and anxiolytic effects. J Funct Foods 2017; 35:105-14. doi: 10.1016/j.jff.2017.05.034 [Crossref] [ Google Scholar]
- Lupien SJ, Juster RP, Raymond C, Marin MF. The effects of chronic stress on the human brain: from neurotoxicity, to vulnerability, to opportunity. Front Neuroendocrinol 2018; 49:91-105. doi: 10.1016/j.yfrne.2018.02.001 [Crossref] [ Google Scholar]
- Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: a review. Excli j 2017; 16:1057-72. doi: 10.17179/excli2017-480 [Crossref] [ Google Scholar]
- Lapp HE, Bartlett AA, Hunter RG. Stress and glucocorticoid receptor regulation of mitochondrial gene expression. J Mol Endocrinol 2019; 62(2):R121-R8. doi: 10.1530/jme-18-0152 [Crossref] [ Google Scholar]
- Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 2015; 22(1-2):6-19. doi: 10.1159/000362736 [Crossref] [ Google Scholar]
- Gray JD, Kogan JF, Marrocco J, McEwen BS. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat Rev Endocrinol 2017; 13(11):661-73. doi: 10.1038/nrendo.2017.97 [Crossref] [ Google Scholar]
- Nade VS, Yadav AV. Anti-stress effect of ethyl acetate soluble fraction of Morus alba in chronic restraint stress. Pharm Biol 2010; 48(9):1038-46. doi: 10.3109/13880200903473741 [Crossref] [ Google Scholar]
- Xiao X, Shang X, Zhai B, Zhang H, Zhang T. Nicotine alleviates chronic stress-induced anxiety and depressive-like behavior and hippocampal neuropathology via regulating autophagy signaling. Neurochem Int 2018; 114:58-70. doi: 10.1016/j.neuint.2018.01.004 [Crossref] [ Google Scholar]
- Samarghandian S, Azimi-Nezhad M, Borji A, Samini M, Farkhondeh T. Protective effects of carnosol against oxidative stress induced brain damage by chronic stress in rats. BMC Complement Altern Med 2017; 17(1):249. doi: 10.1186/s12906-017-1753-9 [Crossref] [ Google Scholar]
- Lenferink LIM, Liddell BJ, Byrow Y, O’Donnell M, Bryant RA, Mau V. Course and predictors of posttraumatic stress and depression longitudinal symptom profiles in refugees: a latent transition model. J Psychiatr Res 2022; 146:1-10. doi: 10.1016/j.jpsychires.2021.12.009 [Crossref] [ Google Scholar]
- Hammen CL. Stress and depression: old questions, new approaches. Curr Opin Psychol 2015; 4:80-5. doi: 10.1016/j.copsyc.2014.12.024 [Crossref] [ Google Scholar]
- Larkin KT, Chantler PD. Stress, depression, and cardiovascular disease. In: Chantler PD, Larkin KT, eds. Cardiovascular Implications of Stress and Depression. Academic Press; 2020. p. 1-12. 10.1016/b978-0-12-815015-3.00001-5.
- Ghanbari Afra L, Zaheri A. Relationship of anxiety, stress, and depression with spiritual health in patients with acute coronary artery disease. J Educ Community Health 2017; 4(2):28-34. doi: 10.21859/jech.4.2.28 [Crossref] [ Google Scholar]
- Lee SP, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. The effect of emotional stress and depression on the prevalence of digestive diseases. J Neurogastroenterol Motil 2015; 21(2):273-82. doi: 10.5056/jnm14116 [Crossref] [ Google Scholar]
- Cañas-González B, Fernández-Nistal A, Ramírez JM, Martínez-Fernández V. Influence of stress and depression on the immune system in patients evaluated in an anti-aging unit. Front Psychol 2020; 11:1844. doi: 10.3389/fpsyg.2020.01844 [Crossref] [ Google Scholar]
- Vieira IS, Ferrugem SCR, Reyes AN, Branco JC, Mondin TC, de Azevedo Cardoso T. Effects of depression and excess body weight on cognition and functioning in young adults: a population-based study. J Affect Disord 2021; 282:401-6. doi: 10.1016/j.jad.2020.12.083 [Crossref] [ Google Scholar]
- Chang JC, Chao YC, Chang HS, Wu YL, Chang HJ, Lin YS, et al. Nose‐to‐brain delivery of mitochondria for treatment of Parkinson’s disease model rats with 6-hydroxydopamine. Res Sq [Preprint]. October 28, 2020. Available from: https://www.researchsquare.com/article/rs-96275/v1.
- Li H, Ge S, Greene B, Dunbar-Jacob J. Depression in the context of chronic diseases in the United States and China. Int J Nurs Sci 2019; 6(1):117-22. doi: 10.1016/j.ijnss.2018.11.007 [Crossref] [ Google Scholar]
- Kotani M, Urushino N, Natsutani I, Ogi Y, Ikeda K. Effects of the 5-HT1A receptor agonists buspirone and 8-OH-DPAT on pupil size in common marmosets. Behav Pharmacol 2017; 28(4):313-7. doi: 10.1097/fbp.0000000000000275 [Crossref] [ Google Scholar]
- Tiwari S, Kusmariya BS, Tiwari A, Pathak V, Mishra AP. Acoustical and viscometric studies of buspirone hydrochloride with cobalt(II) and copper(II) ions in aqueous medium. J Taibah Univ Sci 2017; 11(1):101-9. doi: 10.1016/j.jtusci.2015.10.012 [Crossref] [ Google Scholar]
- Strawn JR, Mills JA, Cornwall GJ, Mossman SA, Varney ST, Keeshin BR. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol 2018; 28(1):2-9. doi: 10.1089/cap.2017.0060 [Crossref] [ Google Scholar]
- Bijak M. Silybin, a major bioactive component of milk thistle (Silybum marianum L Gaernt)-chemistry, bioavailability, and metabolism. Molecules 2017; 22(11):1942. doi: 10.3390/molecules22111942 [Crossref] [ Google Scholar]
- Thakare VN, Patil RR, Oswal RJ, Dhakane VD, Aswar MK, Patel BM. Therapeutic potential of silymarin in chronic unpredictable mild stress induced depressive-like behavior in mice. J Psychopharmacol 2018; 32(2):223-35. doi: 10.1177/0269881117742666 [Crossref] [ Google Scholar]
- Wang X, Zhang Z, Wu SC. Health benefits of Silybum marianum: phytochemistry, pharmacology, and applications. J Agric Food Chem 2020; 68(42):11644-64. doi: 10.1021/acs.jafc.0c04791 [Crossref] [ Google Scholar]
- Haddadi R, Mohajjel Nayebi A, Farajniya S, Eyvari Brooshghalan S, Sharifi H. Silymarin improved 6-OHDA-induced motor impairment in hemi-parkisonian rats: behavioral and molecular study. Daru 2014; 22(1):38. doi: 10.1186/2008-2231-22-38 [Crossref] [ Google Scholar]
- Haddadi R, Mohajjel Nayebi A, Eyvari Brooshghalan S. Pre-treatment with silymarin reduces brain myeloperoxidase activity and inflammatory cytokines in 6-OHDA hemi-parkinsonian rats. Neurosci Lett 2013; 555:106-11. doi: 10.1016/j.neulet.2013.09.022 [Crossref] [ Google Scholar]
- Haddadi R, Mohajjel Nayebi A, Eyvari Brooshghalan S. Silymarin prevents apoptosis through inhibiting the Bax/caspase-3 expression and suppresses toll like receptor-4 pathway in the SNc of 6-OHDA intoxicated rats. Biomed Pharmacother 2018; 104:127-36. doi: 10.1016/j.biopha.2018.05.020 [Crossref] [ Google Scholar]
- Haddadi R, Eyvari Brooshghalan S, Farajniya S, Mohajjel Nayebi A, Sharifi H. Short-term treatment with silymarin improved 6-OHDA-induced catalepsy and motor imbalance in hemi-parkisonian rats. Adv Pharm Bull 2015; 5(4):463-9. doi: 10.15171/apb.2015.063 [Crossref] [ Google Scholar]
- Abbasi BH, Khan MA, Mahmood T, Ahmad M, Chaudhary MF, Khan MA. Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult 2010; 101(3):371-6. doi: 10.1007/s11240-010-9692-x [Crossref] [ Google Scholar]
- El-Elimat T, Alzoubi KH, AbuAlSamen MM, Al Subeh ZY, Graf TN, Oberlies NH. Silymarin prevents memory impairments, anxiety, and depressive-like symptoms in a rat model of post-traumatic stress disorder. Planta Med 2019; 85(1):32-40. doi: 10.1055/a-0710-5673 [Crossref] [ Google Scholar]
- Thakare VN, Aswar MK, Kulkarni YP, Patil RR, Patel BM. Silymarin ameliorates experimentally induced depressive like behavior in rats: Involvement of hippocampal BDNF signaling, inflammatory cytokines and oxidative stress response. Physiol Behav 2017; 179:401-10. doi: 10.1016/j.physbeh.2017.07.010 [Crossref] [ Google Scholar]
- Freitas AE, Bettio LE, Neis VB, Santos DB, Ribeiro CM, Rosa PB. Agmatine abolishes restraint stress-induced depressive-like behavior and hippocampal antioxidant imbalance in mice. Prog Neuropsychopharmacol Biol Psychiatry 2014; 50:143-50. doi: 10.1016/j.pnpbp.2013.12.012 [Crossref] [ Google Scholar]
- Kumar A, Kaur G, Rinwa P. Buspirone along with melatonin attenuates oxidative damage and anxiety-like behavior in a mouse model of immobilization stress. Chin J Nat Med 2014; 12(8):582-9. doi: 10.1016/s1875-5364(14)60089-3 [Crossref] [ Google Scholar]
- Thakare VN, Dhakane VD, Patel BM. Potential antidepressant-like activity of silymarin in the acute restraint stress in mice: modulation of corticosterone and oxidative stress response in cerebral cortex and hippocampus. Pharmacol Rep 2016; 68(5):1020-7. doi: 10.1016/j.pharep.2016.06.002 [Crossref] [ Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985; 14(3):149-67. doi: 10.1016/0165-0270(85)90031-7 [Crossref] [ Google Scholar]
- Mahmoudi J, Farhoudi M, Talebi M, Sabermarouf B, Sadigh-Eteghad S. Antidepressant-like effect of modafinil in mice: evidence for the involvement of the dopaminergic neurotransmission. Pharmacol Rep 2015; 67(3):478-84. doi: 10.1016/j.pharep.2014.11.005 [Crossref] [ Google Scholar]
- Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 2015; 10(2):e0117503. doi: 10.1371/journal.pone.0117503 [Crossref] [ Google Scholar]
- Solati J, Yaghmaei P, Mohammdadi K. Role of the 5-HT1A serotonergic system in anxiolytic-like effects of silymarin. Neurophysiology 2012; 44(1):49-55. doi: 10.1007/s11062-012-9266-0 [Crossref] [ Google Scholar]
- Bandelow B, Reitt M, Röver C, Michaelis S, Görlich Y, Wedekind D. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol 2015; 30(4):183-92. doi: 10.1097/yic.0000000000000078 [Crossref] [ Google Scholar]
- Sadler AM, Bailey SJ. Repeated daily restraint stress induces adaptive behavioural changes in both adult and juvenile mice. Physiol Behav 2016; 167:313-23. doi: 10.1016/j.physbeh.2016.09.014 [Crossref] [ Google Scholar]
- Shan Y, Wang DD, Xu YX, Wang C, Cao L, Liu YS. Aging as a precipitating factor in chronic restraint stress-induced tau aggregation pathology, and the protective effects of rosmarinic acid. J Alzheimers Dis 2016; 49(3):829-44. doi: 10.3233/jad-150486 [Crossref] [ Google Scholar]
- Zhu Y, Klomparens EA, Guo S, Geng X. Neuroinflammation caused by mental stress: the effect of chronic restraint stress and acute repeated social defeat stress in mice. Neurol Res 2019; 41(8):762-9. doi: 10.1080/01616412.2019.1615670 [Crossref] [ Google Scholar]
- Ans AH, Anjum I, Satija V, Inayat A, Asghar Z, Akram I. Neurohormonal regulation of appetite and its relationship with stress: a mini literature review. Cureus 2018; 10(7):e3032. doi: 10.7759/cureus.3032 [Crossref] [ Google Scholar]
- Shireen E, Haleem DJ. Motor effects of buspirone: relationship with dopamine and serotonin in the striatum. J Coll Physicians Surg Pak 2005; 15(12):753-6. [ Google Scholar]
- Chugani DC, Chugani HT, Wiznitzer M, Parikh S, Evans PA, Hansen RL, et al. Efficacy of low-dose buspirone for restricted and repetitive behavior in young children with autism spectrum disorder: a randomized trial. J Pediatr 2016;170:45-53.e4. 10.1016/j.jpeds.2015.11.033.
- Horváth J, Barkóczi B, Müller G, Szegedi V. Anxious and nonanxious mice show similar hippocampal sensory evoked oscillations under urethane anesthesia: difference in the effect of buspirone. Neural Plast 2015; 2015:186323. doi: 10.1155/2015/186323 [Crossref] [ Google Scholar]
- Helton SG, Lohoff FW. Serotonin pathway polymorphisms and the treatment of major depressive disorder and anxiety disorders. Pharmacogenomics 2015; 16(5):541-53. doi: 10.2217/pgs.15.15 [Crossref] [ Google Scholar]
- Muley MM, Thakare VN, Patil RR, Bafna PA, Naik SR. Amelioration of cognitive, motor and endogenous defense functions with silymarin, piracetam and protocatechuic acid in the cerebral global ischemic rat model. Life Sci 2013; 93(1):51-7. doi: 10.1016/j.lfs.2013.05.020 [Crossref] [ Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol 2016; 6(2):603-21. doi: 10.1002/cphy.c150015 [Crossref] [ Google Scholar]
- Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev 2012; 32(4):301-15. doi: 10.1016/j.cpr.2012.02.002 [Crossref] [ Google Scholar]
- Kinlein SA, Wilson CD, Karatsoreos IN. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry 2015; 6:31. doi: 10.3389/fpsyt.2015.00031 [Crossref] [ Google Scholar]
- Gjerstad JK, Lightman SL, Spiga F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018; 21(5):403-16. doi: 10.1080/10253890.2018.1470238 [Crossref] [ Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016; 41(1):3-23. doi: 10.1038/npp.2015.171 [Crossref] [ Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience 2003; 119(3):887-97. doi: 10.1016/s0306-4522(03)00105-2 [Crossref] [ Google Scholar]
- Guedri K, Frih H, Chettoum A, Rouabhi R. Chronic restraint stress induced neurobehavioral alterations and histological changes in rat. Toxicol Environ Health Sci 2017; 9(2):123-9. doi: 10.1007/s13530-017-0312-6 [Crossref] [ Google Scholar]