Biomed Res Bull. 1(1):11-14.
doi: 10.34172/biomedrb.2023.04
Original Article
Angiopoietin 4 Decreases TLR Expression on Monocytes in Multiple Sclerosis Patients
Samira Vedadi 1  , Mohammad Azimzadeh 2, Amir Ebrahimpour Touluei 2, Sona Abolhasani 3, *
, Mohammad Azimzadeh 2, Amir Ebrahimpour Touluei 2, Sona Abolhasani 3, * 
Author information:
1Department of Immunology, Faculty of Medicine, Islamic Azad University of Tabriz Branch, Tabriz, Iran
2Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Neurology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Multiple sclerosis (MS) is a neurodegenerative disease in which myelin sheaths are under the attack of T cells. Angiopoietin 4 is a tyrosine kinase receptor that is expressed and activated in endothelial cells and some hematopoietic cells during development. Normal angiogenesis relies on this receptor. Toll-like receptors (TLRs) are sensitive receptors with the capability of establishing signals toward pathogen-associated molecular patterns.
Methods:
Thirty MS patients were referred to an MS clinic in Imam Reza hospital with different stages ranging from mild to moderate and acute choices. Right after phlebotomy monocytes were isolated from peripheral blood mononuclear cells (PBMCs). Flow cytometry and CD14 were used to evaluate monocyte purity percentage, and cultured monocytes were treated with angiopoietin 4 protein. After stimulation, all cells were gathered, and real-time polymerase chain reaction (PCR) declared the amount of TLR2 and TLR4 activeness.
Results:
The findings revealed the fact that time has a determinative effect on the TLR2 and TLR4 activity levels. Twelve hours of time-lapse right after stimulation resulted in the minimum amount of decrease in TLR2 and TLR4. Comparing TLR2 and TLR4, it was noted that they both exhibited the same amount of decrease. Further, angiopoietin 4’s function was estimated by means of the time course.
Conclusion:
Real-time PCR revealed that angiopoietin 4 has a considerable effect on the decrease of immune receptors such as TLR2 and TLR4.
Keywords: Toll-like receptor, Angiopoietin 4, Multiple sclerosis
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Multiple sclerosis (MS) is an inflammatory pathological condition, the main function of which is to disrupt myelin sheaths on neurons in the brain and spinal cords. This trauma affects the ability of many cells responsible for communication in the nervous system, leading to the emergence of many physical symptoms.1-2 Angiopoietin 4 is a peptide containing 43 amino acids found in all cells except red blood cells. This peptide plays a vital role in repairing damaged skin, eyes, heart, and nerve tissues. Many more biological activities can be done via angiopoietin 4 such as the inhibition of endothelial cell migration, the reduction in inflammatory responses, the inhibition of apoptosis, and oxidative damage. This peptide has an important part in angiogenesis inhabitation, and reacting with actin monomers can be a neurologic factor.3,4
Toll-like receptors (TLRs) are innate immune system receptors that were detected in Drosophila fly for the first time. These receptors can be found in many immune cells such as natural killer cells, mast cells, B cells, regulating T cells, macrophages, dendritic cells, neutrophils, basophils, monocytes, and non-immunity cells such as epithelial and endothelial cells.4 TLRs play a major role in inflammation, and their influence on autoimmune diseases has been approved.5-7 It was thought that TLRs are restricted to microglia, astrocytes, and oligodendrocytes, but it has been confirmed that these receptors are not only in red blood cells, epithelial tissues, endothelial cells, and the brain but also in neurons. The importance of TLR2, TLR4, and TLR9 in message transmission on cellular and membrane surfaces is the main reason to choose these receptors. The purpose of this study was to learn/figure out the inhibitory effect of angiopoietin 4 on the expression of TLR2, TLR4, and TLR9.1,8 Angiopoietin 4 may have an inhibitory effect on the expression levels of the monocytes in MS patients. However, the theoretical aspects of the relationship between angiopoietin 4 and TLR in MS have not been explained.
Materials and Methods
Sample Collection
Samples were collected from MS patients who were referred to the Imam Reza hospital neurology clinic. Thirty MS patients with moderate to severe stages were selected, ranging from 17 to 40 years. The patients used fingolimod and interferon beta as medicine. Blood samples were taken from these patients and monocytes were isolated from peripheral blood mononuclear cells (PBMCs) by the magnetic-activated cell sorting method. MS patients who have been treated for two years using fingolimod and interferon beta were included and patients who were pregnant, patients who discontinued taking the medicine in any way, patients who have been recently diagnosed, and vulnerable people were excluded from the study. The patients who have used fingolimod and interferon beta as a medicine and MS patients who have been treated for two years using fingolimod and interferon beta were included.
Cell Culture
To evaluate the function of angiopoietin 4, four monocytes in the blood were isolated and cultured by the PBMC method. In addition to the magnetic-activated cell sorting method, the purified cells were examined microscopically for the required cell count, survival, and CD14 expression, which indicates the purity of the monocytes. Cells were cultured in Dulbecco’s Modified Eagle Medium. These cells were treated with 10 µL of angiopoietin 4 for 2, 4, 12, 24, and 48 hours.
The Isolation of mRNA and Real-Time Polymerase Chain Reaction
Total RNA was isolated from the monocyte cells from PBMC. The polymerase chain reaction (PCR) was performed with a 1 µL, 1 µL of 5 mM dNTP, 2.5 µL of Taq polymerase, 1.2 µL of 50 mM MgCl2, and 1 µL of each primer. The PCR condition was as follows: initial denaturation at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 40 minutes, and a final elongation cycle at 72°C for 10 minutes (Corbett 6000 Real-Time PCR). Beta-actin was used as a control.
Statistical Analysis
Data were summarized as mean ± standard deviation. The statistical analysis of the results was performed by the one-way analysis of variance, and P values less than 0.05 were considered significant. Statistical analysis was conducted using GraphPad Prism 7 to compare the expression of genes.
Results
Decreased Expression of TLR2, TLR4, and TLR9 by the Stimulation of Angiopoietin 4
The monocyte cells were treated with angiopoietin 4 as a time course (2,4,12,24, and 48 hours), and TLRs’ expression was determined by real-time PCR. The results showed that the expression of TLR2 decreased significantly 12 hours after stimulating with angiopoietin 4, and the same results were observed for both TLR4 and TLR9 (P < 0.0001). The B-ACTIN gene was used as an internal control in this test.
The Effect of Angiopoietin 4 on the Expression of TLR2, TLR4, and TLR9 in Mononuclear Cells of MS Patients Using Fingolimod and Interferon
To investigate the effect of both proteins on the expression of TLR2 and TLR9, their efficiency on the receptor pathways of the innate immune system has been studied. The results indicated that the expression of TLR2 and TLR4 receptors decreased significantly with the time course of stimulation, which reached its lowest level in patients using fingolimod compared to beta interferon (Figure 1).
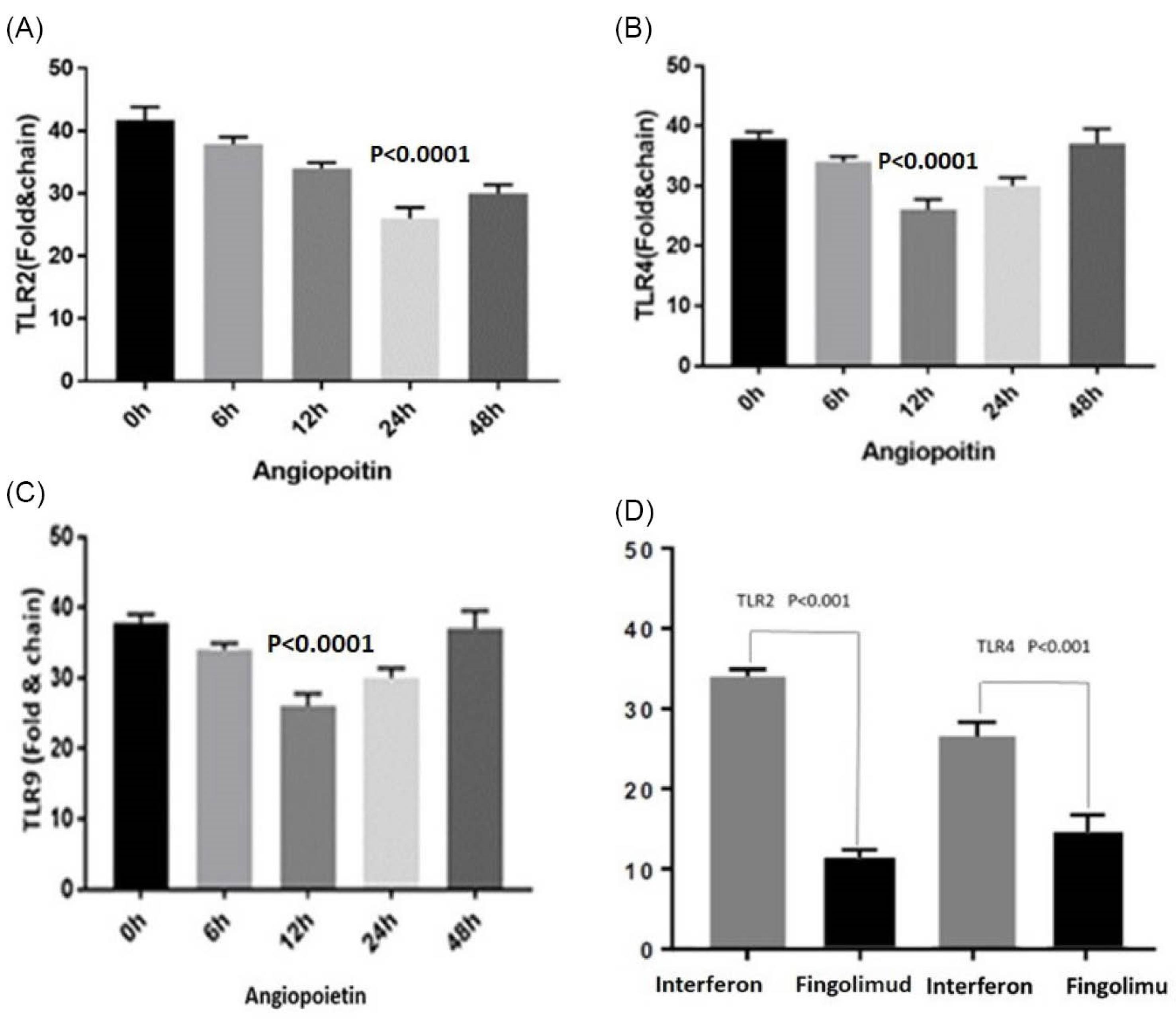
Figure 1.
The Expression of TLRs by treating the monocyte cells with angiopoietin 4 (A) TLR2 expression decreased significantly after 24 hours of treatment with angiopoietin 4; (B) TLR4 expression decreased significantly after 12 hours of treatment with angiopoietin 4; (C) TLR9 expression decreased significantly after 12 hours of treatment with angiopoietin 4; and (D) The expression of TLR2 and TLR4 with inpatient who used the fingolimod and interferon drugs (The TLR9 results did not work properly). Note. TLR: Toll-like receptor
.
The Expression of TLRs by treating the monocyte cells with angiopoietin 4 (A) TLR2 expression decreased significantly after 24 hours of treatment with angiopoietin 4; (B) TLR4 expression decreased significantly after 12 hours of treatment with angiopoietin 4; (C) TLR9 expression decreased significantly after 12 hours of treatment with angiopoietin 4; and (D) The expression of TLR2 and TLR4 with inpatient who used the fingolimod and interferon drugs (The TLR9 results did not work properly). Note. TLR: Toll-like receptor
Discussion
MS is common in people living far from the equator, although there are some exceptions. These cases include ethnic groups that are less likely to get infected such as American Indians, Maori New Zealand, and Inuit Canada. Some groups which live close to the equator are at risk of getting infected such as Palestinians. The reason for this pattern of geographical prevalence is not clear. Several studies showed that people who have traveled to different parts of the world before the age of fifteen have the same rate of infection as the new region, but if the migration occurs after the age of fifteen, the rate of infection will be equal to the rate of the country in which the person was born. These facts indicate that environmental factors during childhood can affect people’s infection with MS.
Diseases such as MS with its complexities have posed a great challenge to researchers. MS is an inflammatory autoimmune disease that originates from the nervous system and affects most of the young people who make up the community of the workforce. Although many cells such as dendritic cells, mast cells, macrophages, and the like can affect this pathological condition, myelin-specific T CD4 autoreactive cells play a more important role in the pathogenesis of this disease.8 Th17, which secrete IL17, plays a key role in the progression of the disease, but regulatory T cells are associated with reducing inflammation.9 The history of MS indicates that this disease has been treated by different doctors in various ways about a century ago.8
Perhaps the most important reason for these different treatments has been doctors’ thinking about the cause of the disease, but over time, in the twentieth century, studies revealed that MS was an autoimmune disease. Some studies have demonstrated that although the number of T lymphocytes is normal in MS patients, they are functionally defective, and Treg cells in MS patients have less power to suppress IL-17 production compared to healthy individuals. Some studies showed a decrease in the number of immune cells in MS patients.6 Recent studies displayed that TLR2 expression increases in patients with MS.10-14 Increased expression is associated with the dysfunction of lymphocyte regulatory cells that can lead to disease.15-20 In another study, several studies found that… found that TLR9 expression significantly increased in MS patients compared with the healthy control group.21-28 A study by Papatheodorou et al showed that although the abundance and inhibitory function of innate immune cells decreases in the peripheral blood of the patient with MS, the abundance of these cells in the cerebrospinal fluid increases.29 In a study done by Zheng et al, it was proved that the percentage of the Th17 cells in the blood samples of patients with active MS was about 7 times higher than that in the healthy control group.30 In Hamid and colleagues’ study, by examining the TLR4 gene in pulmonary patients, it was found that an increase in the stimulation of this gene resulted in the increase in the symptoms and severity of the disease.31 Based on practical background, Tao et al and Wasko et al concluded that this gene activates pro-inflammatory cells by studying the effect of TLLR2 and TLR9 on brain inflammation following stroke.32,33 The exact cause of this disease is not fully understood, but the overproduction of pro-inflammatory cytokines and metalloproteinase by synovitis, macrophages, and monocytes plays an important role in the development and exacerbation of this disease.15
Conclusion
Based on the experiments such as real-time PCR and previous studies, we concluded that the expression of TLR2 and TLR4 receptors decreased significantly with the time course of stimulation over time, which reached the lowest level after stimulation with angiopoietin 4. Each type of disease has its difficulties and problems. The responsibility of all people, including medical students, professors, and medical staff, is to free the world from disease and suffering. Different treatments and different ways of thinking have led us to be hopeful and try to keep our beloved ones and people safe and free from any illness.
Competing interests
Authors declare that they have no competing interests.
Ethical Approval
The study was approved by the Ethics Committee of Islamic Azad University, Tabriz (IR.IAU.TABRIZ.REC.1399.088).
References
- Barreiro-Alonso A, Lamas-Maceiras M, García-Díaz R, Rodríguez-Belmonte E, Yu L, Pardo M. Delineating the HMGB1 and HMGB2 interactome in prostate and ovary epithelial cells and its relationship with cancer. Oncotarget 2018; 9(27):19050-64. doi: 10.18632/oncotarget.24887 [Crossref] [ Google Scholar]
- Barreiro-Alonso A, Cámara-Quílez M, Salamini-Montemurri M, Lamas-Maceiras M, Vizoso-Vázquez Á, Rodríguez-Belmonte E. Characterization of HMGB1/2 interactome in prostate cancer by yeast two hybrid approach: potential pathobiological implications. Cancers (Basel) 2019; 11(11):1729. doi: 10.3390/cancers11111729 [Crossref] [ Google Scholar]
- Barreiro-Alonso A. Interactome of Ixr1, HMGB1 and HMGB2 Proteins in Relation to Their Cellular Functions [thesis]. Universidade da Coruña; 2017.
- VanPatten S, Al-Abed Y. High mobility group box-1 (HMGb1): current wisdom and advancement as a potential drug target. J Med Chem 2018; 61(12):5093-107. doi: 10.1021/acs.jmedchem.7b01136 [Crossref] [ Google Scholar]
- Mammana S, Fagone P, Cavalli E, Basile MS, Petralia MC, Nicoletti F, et al. The role of macrophages in neuroinflammatory and neurodegenerative pathways of Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis: pathogenetic cellular effectors and potential therapeutic targets. Int J Mol Sci 2018;19(3). 10.3390/ijms19030831.
- Li T, Gao Q, Luan G. HMGB1-TLR signaling in Rasmussen’s encephalitis. J Neuroinfect Dis 2016; 7(3):223. doi: 10.4172/2314-7326.1000223 [Crossref] [ Google Scholar]
- Sormani MP. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol 2020; 19(6):481-2. doi: 10.1016/s1474-4422(20)30147-2 [Crossref] [ Google Scholar]
- Dobson R, Giovannoni G. Multiple sclerosis-a review. Eur J Neurol 2019; 26(1):27-40. doi: 10.1111/ene.13819 [Crossref] [ Google Scholar]
- Lassmann H. Multiple sclerosis pathology. Cold Spring Harb Perspect Med 2018;8(3). 10.1101/cshperspect.a028936.
- Motl RW, Sandroff BM, Kwakkel G, Dalgas U, Feinstein A, Heesen C. Exercise in patients with multiple sclerosis. Lancet Neurol 2017; 16(10):848-56. doi: 10.1016/s1474-4422(17)30281-8 [Crossref] [ Google Scholar]
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17(2):162-73. doi: 10.1016/s1474-4422(17)30470-2 [Crossref] [ Google Scholar]
- Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81(6):857-70. doi: 10.1002/ana.24954 [Crossref] [ Google Scholar]
- Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet 2017; 389(10076):1336-46. doi: 10.1016/s0140-6736(16)30959-x [Crossref] [ Google Scholar]
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376(3):221-34. doi: 10.1056/NEJMoa1601277 [Crossref] [ Google Scholar]
- Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: clinical aspects. Curr Opin Neurol 2018; 31(6):752-9. doi: 10.1097/wco.0000000000000622 [Crossref] [ Google Scholar]
- Azami M, YektaKooshali MH, Shohani M, Khorshidi A, Mahmudi L. Epidemiology of multiple sclerosis in Iran: a systematic review and meta-analysis. PLoS One 2019; 14(4):e0214738. doi: 10.1371/journal.pone.0214738 [Crossref] [ Google Scholar]
- Moosazadeh M, Esmaeili R, Mehdi Nasehi M, Abedi G, Afshari M, Farshidi F. Prevalence of familial multiple sclerosis in Iran: a systematic review and meta-analysis. Iran J Neurol 2017; 16(2):90-5. [ Google Scholar]
- Fani Maleki A, Rivest S. Innate immune cells: monocytes, monocyte-derived macrophages and microglia as therapeutic targets for Alzheimer’s disease and multiple sclerosis. Front Cell Neurosci 2019; 13:355. doi: 10.3389/fncel.2019.00355 [Crossref] [ Google Scholar]
- Barry A, Cronin O, Ryan AM, Sweeney B, Yap SM, O’Toole O. Impact of exercise on innate immunity in multiple sclerosis progression and symptomatology. Front Physiol 2016; 7:194. doi: 10.3389/fphys.2016.00194 [Crossref] [ Google Scholar]
- Silva BA, Leal MC, Farías MI, Avalos JC, Besada CH, Pitossi FJ. A new focal model resembling features of cortical pathology of the progressive forms of multiple sclerosis: influence of innate immunity. Brain Behav Immun 2018; 69:515-31. doi: 10.1016/j.bbi.2018.01.010 [Crossref] [ Google Scholar]
- Hossain MJ, Tanasescu R, Gran B. Innate immune regulation of autoimmunity in multiple sclerosis: focus on the role of toll-like receptor 2. J Neuroimmunol 2017; 304:11-20. doi: 10.1016/j.jneuroim.2016.12.004 [Crossref] [ Google Scholar]
- Koudriavtseva T, Mainero C. Neuroinflammation, neurodegeneration and regeneration in multiple sclerosis: intercorrelated manifestations of the immune response. Neural Regen Res 2016; 11(11):1727-30. doi: 10.4103/1673-5374.194804 [Crossref] [ Google Scholar]
- Paudel YN, Angelopoulou E, C BK, Piperi C, Othman I. High mobility group box 1 (HMGB1) protein in multiple sclerosis (MS): mechanisms and therapeutic potential. Life Sci 2019; 238:116924. doi: 10.1016/j.lfs.2019.116924 [Crossref] [ Google Scholar]
- Bucova M, Majernikova B, Durmanova V, Cudrakova D, Gmitterova K, Lisa I. HMGB1 as a potential new marker of disease activity in patients with multiple sclerosis. Neurol Sci 2020; 41(3):599-604. doi: 10.1007/s10072-019-04136-3 [Crossref] [ Google Scholar]
- Sun Y, Chen H, Dai J, Wan Z, Xiong P, Xu Y. Glycyrrhizin protects mice against experimental autoimmune encephalomyelitis by inhibiting high-mobility group box 1 (HMGB1) expression and neuronal HMGB1 release. Front Immunol 2018; 9:1518. doi: 10.3389/fimmu.2018.01518 [Crossref] [ Google Scholar]
- Nishioku T, Kawamoto M, Okizono R, Sakai E, Okamoto K, Tsukuba T. Dimethyl fumarate prevents osteoclastogenesis by decreasing NFATc1 expression, inhibiting of erk and p38 MAPK phosphorylation, and suppressing of HMGB1 release. Biochem Biophys Res Commun 2020; 530(2):455-61. doi: 10.1016/j.bbrc.2020.05.088 [Crossref] [ Google Scholar]
- Han Y, Yang L, Liu X, Feng Y, Pang Z, Lin Y. HMGB1/CXCL12-mediated immunity and Th17 cells might underlie highly suspected autoimmune epilepsy in elderly individuals. Neuropsychiatr Dis Treat 2020; 16:1285-93. doi: 10.2147/ndt.s242766 [Crossref] [ Google Scholar]
- Hamid KM, Nejati A, Shoja Z, Mollaei-Kandelousd Y, Doosti R, Mirshafiey A. Quantitative evaluation of BAFF, HMGB1, TLR 4 AND TLR 7 expression in patients with relapsing remitting multiple sclerosis. Iran J Allergy Asthma Immunol 2016; 15(1):75-81. [ Google Scholar]
- Papatheodorou A, Stein A, Bank M, Sison CP, Gibbs K, Davies P. High-mobility group box 1 (HMGB1) is elevated systemically in persons with acute or chronic traumatic spinal cord injury. J Neurotrauma 2017; 34(3):746-54. doi: 10.1089/neu.2016.4596 [Crossref] [ Google Scholar]
- Zheng C, Chen J, Chu F, Zhu J, Jin T. Inflammatory role of TLR-MyD88 signaling in multiple sclerosis. Front Mol Neurosci 2019; 12:314. doi: 10.3389/fnmol.2019.00314 [Crossref] [ Google Scholar]
- Zheng C, Chen J, Chu F, Zhu J, Jin T. Inflammatory Role of TLR-MyD88 Signaling in Multiple Sclerosis. Front Mol Neurosci 2020; 12:314. doi: 10.3389/fnmol.2019.00314 [Crossref] [ Google Scholar]
- Tao Y, Zhang X, Markovic-Plese S. Toll-like receptor (TLR)7 and TLR9 agonists enhance interferon (IFN) beta-1a’s immunoregulatory effects on B cells in patients with relapsing-remitting multiple sclerosis (RRMS). J Neuroimmunol 2016; 298:181-8. doi: 10.1016/j.jneuroim.2016.07.019 [Crossref] [ Google Scholar]
- Wasko NJ, Nichols F, Clark RB. Multiple sclerosis, the microbiome, TLR2, and the hygiene hypothesis. Autoimmun Rev 2020; 19(1):102430. doi: 10.1016/j.autrev.2019.102430 [Crossref] [ Google Scholar]