Biomed Res Bull. 2(1):29-36.
doi: 10.34172/biomedrb.2024.05
Review Article
A Review of Osmotic Pump Applications as a Reliable Drug Delivery System in Pharmaceutical Sciences
Samar Mahari 1  , Seydeh Halimeh Najafi 1
, Seydeh Halimeh Najafi 1  , Salar Masoomzadeh 2
, Salar Masoomzadeh 2  , Shalen Kumar 3, 4
, Shalen Kumar 3, 4  , Tooba Gholikhani 5, 6, *
, Tooba Gholikhani 5, 6, * 
Author information:
1Student Research Committee, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
2Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
3Ra Biotech ltd, Wellington, Wellington region, New Zealand
4Research Committee, School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand
5Department of Pharmaceutics, Faculty of Pharmacy, Islamic Azad University, Amol, Iran
6Nano Ra Pharmaceuticals, Istanbul, Turkey
Abstract
Systems known in the pharmaceutical sciences have less use for drug release and control. In addition, these standard systems in pharmacy have no effective control over drug concentrations. The main problem with conventional drug delivery systems (DDS) is the unpredictability of different plasma concentrations. However, drug control systems have provided an excellent way to release the drug. In this regard, osmotic pumps are the best and most promising systems for the controlled delivery and release of drugs. They are commonly used for oral and injectable use, several of which are also commercialized and widely accepted by patients, especially the antihypertensive products. The purpose of this study is to introduce and compare different types of osmotic pumps in pharmacy.
Keywords: Osmotic pump, Drug release, Pharmacy, Application
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Introduction
For the past decades, many acute and chronic diseases have been treated by making or prescribing different types of drugs.1 In the past, oral medication administration had been considered the most widely used method for systemic drug delivery.2-7 They are still one of the most common systems in drug delivery in which a person cannot maintain the effect of drug concentration for a long time.
Factors such as pH, chemical and physical properties, physiological agents of the drug, and the appropriate temperature may be involved in determining the bioavailability of drugs.8,9
Several new drug release systems have been recently invented,10,11 and the pharmaceutical industry has faced a new pharmaceutical market in the last decade. In addition, large sums of money have been spent on the production of new drugs, with over $800 million allocated to each new drug.10,12
Research into new osmotic systems for controllable drug delivery and finding treatments to prevent and treat various diseases id continued on.13 The oral drug control system demonstrates a specific drug release pattern; it also has the benefits of reducing the dose and targeting ability. The drug concentration is long maintained between the minimum effective concentration and the maximum safe concentration.14,15
In addition to the drug itself, the correct dose of the drug is also significant for an effective treatment. Controlled drug release systems help maintain drug concentrations in the body, finally minimizing the side effects of the drug and thus improving patient compliance.13
Over the years and in recent years, various technologies in pharmacy have been developed to achieve this goal. Further, only a few of these technologies are used for different therapeutic applications.16-19
Oral medications often suffer from poor pharmacokinetics; for example, their absorption in the gastrointestinal tract is prolonged.20,21
By better regulating the secretion of drugs using osmotic systems,15 these new systems and technologies can enhance the properties of various medications.19 In recent years, many efforts have been made to deliver drugs quickly and easily.15 Although the first osmotic pumps were invented 50 years ago, the development of this technology/system has continued until now.22 Different types of osmotic pumps are illustrated in Figure 1.
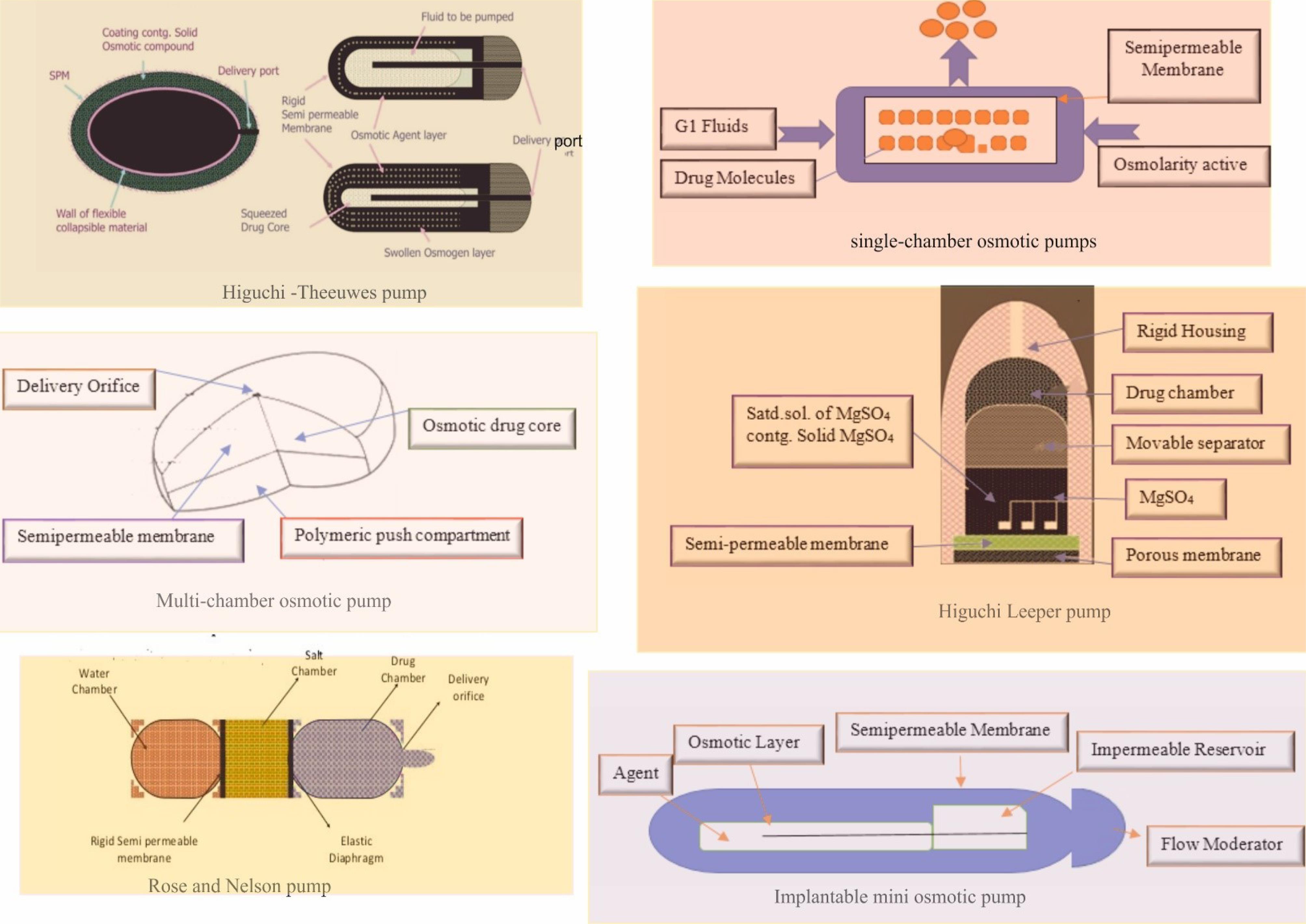
Figure 1.
Different Types of Osmotic Pumps
.
Different Types of Osmotic Pumps
Osmotic systems have several upsides and require no electrical energy to operate. Furthermore, drugs can be stored in liquid or solid form in these pumps. For this reason, osmotic systems are one of the most economical drug delivery systems (DDSs).16,17 Moreover, efficient drug storage can cause drug release for a long time.18,19 Drug release can be modified in a variety of ways,15 but most new DDSs use matrices, reservoirs, or osmosis.23 In matrix systems, the drug is released between the polymer matrix and the external environment.24 Nevertheless, in the reservoir system, there is a drug nucleus that is surrounded by a membrane.
Osmotic systems use osmotic pressure principles to deliver drugs both orally and by injection.23 This study was designed to investigate the applications of osmotic pumps in pharmacy.
History of Osmotic Pump Use in Pharmacy
The osmotic DDSs first became popular among the people in 1748. In 1877, support for this system was provided for the quantitative measurement of osmotic pressure. The first osmotic pump was invented by two Australian pharmacists, Rose and Nelson, in 1955.25
Then, Higuchi and Lipper introduced the Rose and Nelson osmotic pump to the pharmaceutical world with modifications in 1973. In the same year, an osmotic pump distributor was designed with a filler containing osmotic powder.26
Oral osmotic pumps are also used in the gastrointestinal tract treatment system. Finally, the first oral osmotic pump, the elementary osmotic pump, was developed in 1975.
ALZA was granted a US patent in 1976 because they were the ones who developed the oral osmosis pump and are now at the forefront of OROS technology. Initially, the ALZA osmotic pump was only utilized in laboratory animals. The first osmotic drug delivery and release device was developed in 1979.27
In 1982, a patent was issued for an osmotic system containing a liquid hydrogel layer. Moreover, hybrid therapy using high-pressure osmotic pumps was first reported in 1984. Osmotic pumps with controlled conditions were first introduced in 1985 and patented in 1986. Additionally, in 1989, Pfizer Inc. developed a new formulation of the osmotic pump called the Procardia, which was recognized as the most extensive and best-selling cardiovascular product in the United States.
The device contained a capsule containing an active ingredient and an orifice for drug delivery and release. It was surrounded by a semipermeable osmotic active layer.28
In 1999, an asymmetric membrane capsule was invented to deliver and release drugs through osmotic pressure.
In 2000, DUROS Leupold capsules, called Viadur, were recognized in the United States as the first drug-releasing osmotic pump in humans.
The first report of a blue osmotic pump was presented in 2003. This progress proves that drug control through osmotic pump systems is a promising drug release method studied for the past three decades and is still in progress.29 The commercial osmotic pumps are tabulated in Table 1 with their active ingredients, osmotic pump type, and indication.30-33
Table 1.
Commercial Osmotic Pumps
|
Product Name
|
API
|
Dose
|
Type of Osmotic Pump
|
Indication
|
| Cardura XL |
Doxazosin |
4 mg and 8 mg |
Push-pull osmotic pump (bilayer-layer osmotic pump) |
Treatment of hypertension |
| ChronogesicTM |
Sufentanil |
|
Implantable osmotic system |
For long-term pain relief |
| Alpress LP |
Prazosin |
2.5 mg and 5 mg |
Push-pull osmotic pump |
Hypertension treatment |
| Acutrim |
Phenylpropanolamine |
75 mg |
Elementary pump and/or osmotic pump (mono-layer osmotic pump) |
For the treatment of congestion associated with allergies, hay fever, sinus irritation, and the common cold |
| Ditropan XL |
Oxybutynin chloride |
5 mg and 10 mg |
Push-pull osmotic pump |
For once daily overactive bladder treatment with symptoms of urge urinary incontinence, urgency, and frequency |
| Efidac 24 |
Chlorpheniramine maleate |
4 mg IR and 12 mg CR |
Elementary pump and/or osmotic pump |
Used to treat sneezing, runny nose, itching, watery eyes, hives, rashes, itching, and other symptoms of allergies and the common cold |
| Covera HS |
Verapamil |
180 mg and 240 mg |
Push-pull osmotic pump with time delay |
Hypertension and angina |
| Efidac 24 |
Pseudoephiderine |
60 mg IR and 180 mg CR |
Elementary pump and/or osmotic pump |
Temporary relief of stuffy nose and sinus pain/pressure caused by infection or other breathing illnesses |
| Dynacirc CR |
Isradipine |
5 mg and 10 mg |
Push-pull osmotic pump |
Treatment of hypertension |
| Glucotrol XL |
Glipizide |
5 mg and 10 mg |
Push-pull osmotic pump |
For the control of hyperglycemia in patients with non-insulin-dependent diabetes |
| Viadur |
Leuprolide acetate |
72 mg |
Implantable osmotic system |
Treatment of prostate cancer |
| Procadia XL |
Nifedipine |
30 mg, 60 mg, and 90 mg |
Push-pull osmotic pump |
Used for angina, Prinzmetal’s angina, and hypertension |
| Invega |
Paliperidone |
1.5 mg, 3 mg, 6 mg, and 9 mg |
Push-pull osmotic pump |
Treatment of schizophrenia and schizoaffective disorder |
| Minipress XL |
Prazocine |
2.5 and 5 mg |
Elementary osmotic pump |
Treatment of hypertension |
| Volmex |
Albuterol |
4 and 8 mg |
Elementary osmotic pump |
For relief of bronchospasm in patients with reversible obstructive airway disease |
| Procadia XL |
Nifedipine |
30 mg, 60 mg, and 90 mg |
Push-pull osmotic pump |
Treatment of hypertension and angina |
| Sudafed 24 |
Pseudoephedrine |
240 mg |
Elementary osmotic pump |
Used for the temporary relief of stuffy nose and sinus pain/pressure caused by infection or other breathing illnesses |
| Tegretol XR |
Carbamazepine |
100 mg, 200 mg, and 400 mg |
OROS tablet |
Used as an anticonvulsant drug |
| Concerta |
Methylphenidate |
18 mg, 36 mg, 54 mg, and 72 mg |
Implantable osmotic systems |
For the treatment of attention-deficit hyperactivity disorder, postural orthostatic tachycardia syndrome, and narcolepsy |
| Teczem |
Enalapril and Diltiazem |
180/5 mg |
Elementary osmotic pump |
Treatment of hypertension |
| Tiamate |
Diltiazem |
180 mg, 240 mg, and 360 mg |
Push-pull osmotic pump |
Raynaud’s disease, hypertension, supraventricular tachyarrhythmias, and vasospastic angina |
| Allegra D |
Fexofenadine |
30 mg, 60 mg, and 180 mg |
Implantable osmotic systems |
Relieves nasal congestion, sinus pressure, sneezing, itchy, watery eyes, an itchy nose, and an itchy throat |
| Altoprev® |
Lovastatin |
20 mg, 40 mg, and 60 mg |
Elementary osmotic pump with a time delay |
Used to lower the risk of stroke, heart attack, and other heart complications in people with diabetes, coronary heart disease, or other risk factors |
| Cardura® XL |
Doxazosin |
4 and 8 mg |
Push-pull osmotic pump |
Used to treat symptoms of benign prostatic hyperplasia and hypertension |
Note. API: active pharmaceutical ingredient
Osmosis and the Basics of Using Osmotic Pumps in Pharmacy
Water regulation in plants and cells is made possible by a phenomenon called osmosis.34 An osmotic current is generated when two solutions of different concentrations pass through a semipermeable membrane.2,35,36
The tablet has a hard water-permeable coating with one or more small pores created by a laser on its surface.37 As the capsule enters the body, the current causes the solutes to move from low to high concentration.35 This action eventually leads to a difference in hydrostatic pressure in the semipermeable membrane, and the pressure difference balances the osmotic flow and drug release.36,30 The creation of osmotic pressure through the secretion of fluids from the external environment can regulate the release and secretion of drugs in the osmotic system.31 The synergy of solutions is another essential feature of osmotic pressure in which the number of substances dissolved in the solution is entirely independent of osmotic pressure.32 In other words, drug release rates from the osmotic dispensers depend on the appropriate solubility, the appropriate molecular weight, and the activity coefficient of the solution.33
The main components in osmotic systems with pharmaceutical applications are summarized in Figure 2. One of the components is an osmotic or cosmogenic agent, which creates an extremely high osmotic pressure inside the system and increases the drug release rate. Several commercial osmotic agents are implemented in the osmotic system, including sodium chloride, fructose, sucrose, potassium chloride, xylitol, sorbitol, citric acid, dextrose, mannitol, and lactose. Sometimes a mixture of compounds, such as dextrose with fructose/lactose/mannitol/sucrose is used to generate desirable osmotic pressure.
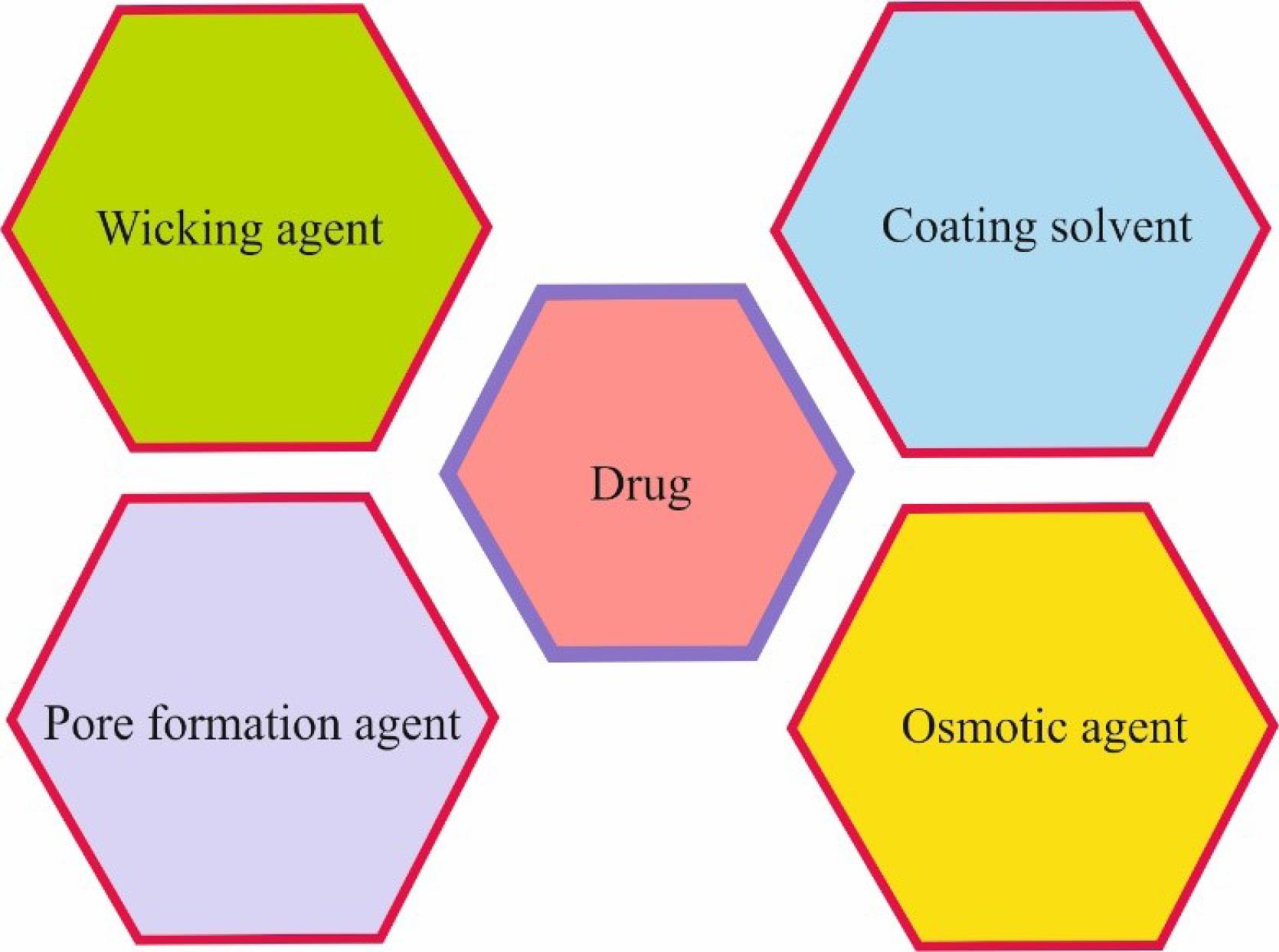
Figure 2.
Main Components in Osmotic Systems With Pharmaceutical Applications
.
Main Components in Osmotic Systems With Pharmaceutical Applications
The other compound that helps speed up the drug release by increasing the drug contact level is the Wicking agent. Colloidal silicon dioxide and sodium lauryl sulfate are of this type; they help the drug enter the aqueous environment.2
Alkaline salts, such as sodium chloride, sodium bromide, potassium chloride, potassium sulfate, and potassium phosphate, are pore-forming agents that form fine-grained membranes.5 The other essential factor is the coating solvent; the primary function of the solvent system is to dissolve or disperse the polymer and other additives and transfer them to the substrate surface. Coating solvents should not damage the core, wall, or other materials. Methylene chloride, methanol, isopropyl alcohol, dichloromethane, ethyl acetate, acetone, carbon tetrachloride, cyclohexane, butyl alcohol, and water are different types of solvents.
Drugs for being used in an osmotic system need to have some unique characteristics, such as not having too low or too high solubility. Additionally, drugs with a biological half-life of more than 12 hours get affected in the long run.4 The best choice for osmotic systems is drugs utilized for the long-term cure of diseases with a biological half-life of 1–6 hours.3 Therefore, diazepam, penicillin G, and furosemide with a half-life of less than 1 hour may not be suitable options for a controlled osmotic release system.4
Osmotic Pump Systems
Rose and Nelson Pump
Two Australian physiologists patented the first osmotic pump in 1955. With this osmotic pump, they injected the drug into the intestines of sheep and cows.38 This type of osmotic pump consists of a separate chamber that includes a medicine chamber, a salt-containing chamber, and a water chamber.38 This pump has a semipermeable membrane that can filter drugs into the water chamber.39 Creating a pressure difference across the membrane causes water to move from the water chamber to the salt chamber.39 The volume of the salt chamber increases due to the difference in osmotic pressure and flow of water between two chambers, resulting in the drug leaving the device.40
Higuchi-Leeper Pump
The Higuchi-Leeper pump is widely used in animals to deliver antibiotics or hormones. The Higuchi Leeper pump has a semipermeable membrane consisting of a highly strong chamber.41 The pump also has a solid layer of low-melting wax; it has microcrystalline paraffin that can be utilized instead of an elastic diaphragm and an osmotic chamber to separate the drug.42 Drug secretion is performed by perforating the elastic material under osmotic pressure.42 Release occurs after reaching high pressure and opening the pores. Then, the pressure closes pores, and the cycle is repeated.41 The pores should be small enough to close quickly in the absence of an osmotic pressure threshold.43
Higuchi Theeuwes Pump
Higuchi and Theeuwes could produce a simpler version of the Rose-Nelson pump in the early 1970s. This pump, similar to the Higuchi-Leeper pump, uses water to activate the osmotic pump.44 In addition, in the Higuchi-Theeuwes machine, the membrane acts as the outer cover of the pump. This membrane is robust and impermeable, with sufficient resistance to the pumping pressure inside the device. This pump is loaded with the desired drug before use.45 When this osmotic pump is placed in an aqueous medium, its drug content is released by the salt chamber through the outer membrane coating. Because of this phenomenon, most Higuchi-Theeuwes pumps use solid salts.45
Implantable Mini-osmotic Pump
The pump consists of three concentric layers, namely, a drug tank, an osmotic sleeve, a semipermeable membrane, and a speed controller. In the body of the osmotic pump, there is an additional component called mud. In this type of pump, the inside of the drug tank chamber is surrounded by an osmotic coating and a high-concentration cylindrical chamber.46 When this osmotic system is placed in an aqueous medium, water enters the pump cover through a semipermeable membrane, in which case the tank becomes flexible, and pressure, which is applied to the drug, moves the drug content.47 In general, the rate of drug delivery through these pumps is between 10 and 0.25 mL per hour, and delivery time is 1–4 weeks.48
Single-chamber Osmotic Pump
In 1974, Theeuwes invented the first osmotic pump, which consisted of an active material with a suitable osmotic pressure. It was a capsule and a semipermeable membrane coated with cellulose acetate.49 When this coated capsule of the drug was placed in an aqueous medium, the osmotic pressure caused the drug to draw water into the capsule through a semipermeable coating to form a saturated drug inside the system.50 As the membrane was not expandable, water accumulation inside the pump increased hydrostatic pressure and eventually created a small hole outside the pump.51
Multi-chamber Osmotic Pump
The multi-chamber osmotic pump is a modified elementary osmotic pump, through which water-soluble drugs can be delivered at a constant speed.52 This system is similar to a layer-by-layer capsule, and the polymerization can make the drug last longer. A layer containing osmotic materials, polymeric materials, and excipients is released when the capsule enters the water. By compression, the capsules join to form a two-layer core.53 A semipermeable membrane covers the core of the tablet. Then, a small hole next to the capsule layers is made using lasers or mechanical drills. When this osmotic system is placed in an aqueous medium, osmotic pressure is created, and water is drawn into the tablet.53 To make a drug suspension, osmotic absorption is created in the drug layer, and water is drawn into chamber.54 The osmotic pressure created in the non-drug layer causes water to enter the drug chamber, increases the volume of the drug layer, and expands the non-drug suspension layer to remove the drug from the pump.54
Osmotically Controlled Drug Delivery System
One of the most promising drug control systems is osmotic pumps, called osmosis water movement in a permeable membrane.55 Osmotic systems are used to expand the drug control system. Osmotic pressures are also utilized to move and release drugs in a controlled manner.52 These systems can be employed in oral and planting methods. The existing osmotic pumps have more benefits than other drug control systems, one of which is their easy formulation. Another advantage is their cheap and easy production.56 Moreover, these systems are suitable for prescribing oral medications.53 They consist of a compressed capsule with a semipermeable membrane and several holes for drug release. The core of this capsule consists of an osmotic agent and a water-soluble polymer.49 The rate of adsorption of this nucleus depends on the osmotic pressure and the permeability of the membrane. When water enters the capsule, the nucleus in it expands, and the drug solution in it leaves the capsule through the existing pores. One of the main differences between these systems and osmotic systems is that they release the drug into the external environment independently of pH and hydrodynamics.57
Osmotic Matrix Systems
It is a type of single-chamber osmotic pump known as an “osmotic matrix system”.55 This osmotic system does not require a separate, semipermeable membrane. In contrast, uniformly dispersed drug particles used as osmotic agents are placed directly in the matrix of the osmotic polymer system as semipermeable membranes.56 The size of this type of particle is less than 40 µm, so that they form a large number of microcapsules throughout matrix.2 Gill et al first introduced the concept. The osmotic mechanism of drug release is as follows56:
Water is dispersed around the polymer matrix, where it encounters drug particles and dissolves them. Water creates an osmotic pressure in the matrix walls. The resulting osmotic pressure causes the soluble drugs to be diluted inside the capsules. Hydrostatic pressures accumulate in each microcapsule until the matrix walls crack. When a crack occurs, the soluble drug leaks through the pores and connects the previously torn microcapsules.2 This mechanism is caused by various factors such as concentration, elastic model, and the like. Matrix osmotic systems are also known as single-chamber pumps that propagate at different times.56 The secretion of lipophilic drugs can be controlled as a Higuchi matrix.56 Devices that use this drug release mechanism contain steroid rings for easy and rapid release of electrodes.32
Duros Technology
DUROS are tiny osmotic implants that release drugs for 3 months to 1 year with precise kinetics. DUROS is a drug system that is suitable for solid drugs and can deliver a maximum of 500 mg of drug from one capsule to a 1 cc drug tank. This technology is an advanced formulation that maximizes the drug load, stabilizes drugs chemically and physically at body temperature for a long time, and includes aqueous and non-aqueous transport devices.27 DUROS technology has many uses in clinical and pre-clinical trials, including the Chronogesic system, which systematically delivers sufentanil for chronic pain.12 This technology is a small drug dispensing system that works similar to a small syringe, releasing small amounts of concentrated drug formulations in a continuous stream over months or years (Figure 3). This system is implanted under the skin and can be as much as 4 mm/L. The drug formulation is present in the drug container chamber. The drug formulation may be in solution or suspension. DUROS medicinal solutions can be aqueous or non-aqueous. Long-term DUROS formulations should show stability in body temperature (37 °C), which usually varies from 3 months to 1 year. The DUROS system has been selected for its biocompatibility and suitability for use in capsules. Drug-contact materials are also screened for compatibility with drugs and specific drug formulations.1 Gamma sterilization (gamma) may be utilized to sterilize the final medicinal product (Table 2). If the drug formulation fails to withstand radiation disinfection doses, a subset of DUROS is destroyed by radiation, and the drug formulation is added in a final aseptic operation.12
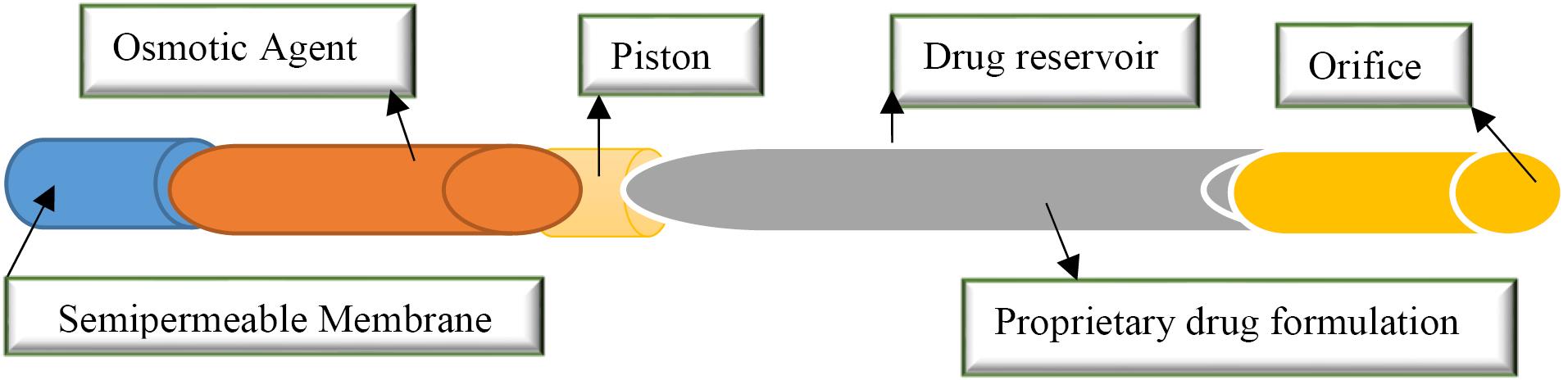
Figure 3.
DUROS Technology
.
DUROS Technology
Table 2.
Comparison of Osmotic Pumps Based on Preparation Architectural Features
|
Type of Osmotic Pump
|
Features
|
| Mono-layer osmotic pump or primary osmotic pump |
-Made up of a monolayer release-controlled film.
-Mainly used in APIs with an intermediate solubility range.
-Has one small orifice.
-The drug is released as a solution.
-The drug release rate is independent of pH and gastrointestinal tract motility patterns. |
| Bilayer or “push-pull” osmotic pumps |
-Made up of two layers.
-The upper layer is a medicated layer.
-The lower one is the push layer, which prevents insoluble medicine from residing in a pump chamber for a long time.
-Osmotic agent presents in both layers.
-Has one or more small orifices.
-The drug is released as a solution or suspension.
-Suitable for extremely soluble or insoluble active agents.
-The drug release rate is independent of pH and gastrointestinal tract motility patterns. |
| Three- or multi-layered osmotic pump (push-stick osmotic pump) |
-Has three layers.
-Has one non-pastille push layer.
- Has one medicine layer.
- Has one pharmaceutical pack coating.
-Has one large orifice.
-The drug is released as a wet mass that requires subsequent disintegration and dissolution. |
| L-OROS |
- Has a single softgel capsule.
- Has one small orifice.
-The drug is released as a liquid. |
| Single-composition osmotic tablet |
- Has a single-layer tablet.
- Has no predrilled orifice.
-The drug is released as a solution or wet mass through channels or cracks formed in situ. |
Conclusion
Osmotic pumps in pharmaceutical systems have become highly widespread in recent years. Today, due to new technologies in the pharmaceutical system, many DDSs based on osmotic systems are well-known in the pharmaceutical sciences. Different forms of drug delivery have less control over drug secretion and have no control over the effective concentration of the drug at the target site. Simultaneously, osmotic pump systems can release the drug more rapidly and in a controlled manner. It seems that among different architectures implemented in the development of the elementary and push-pull osmotic pumps were more commercialized ones than others, most of which were used for the delivery of antihypertensive agents.
Authors’ Contribution
Conceptualization: Shalen Kumar.
Data curation: Samar Mahari.
Methodology: Tooba Gholikhani.
Project administration: Halimeh Najafi.
Resources: Samar MAhari.
Supervision: Tooba Gholikhani.
Visualization: Samar Mahari.
Writing–original draft: Samar Mahari, Halimeh Najafi.
Writing–review & editing: Tooba Gholikhani.
Competing Interests
The authors declare no conflict of interests.
Ethical Approval
Not applicable.
References
- Sahoo CK, Sahoo NK, Rao SR, Sudhakar M, Satyanarayana K. A review on controlled porosity osmotic pump tablets and its evaluation. Bull Fac Pharm Cairo Univ 2015; 53(2):195-205. doi: 10.1016/j.bfopcu.2015.10.004 [Crossref] [ Google Scholar]
- Cheng X, Sun M, Gao Y, Cao F, Zhai G. Design and evaluation of osmotic pump-based controlled release system of ambroxol hydrochloride. Pharm Dev Technol 2011; 16(4):392-9. doi: 10.3109/10837451003774385 [Crossref] [ Google Scholar]
- Zhao Z, Wu C, Zhao Y, Hao Y, Liu Y, Zhao W. Development of an oral push-pull osmotic pump of fenofibrate-loaded mesoporous silica nanoparticles. Int J Nanomedicine 2015; 10:1691-701. doi: 10.2147/ijn.s76755 [Crossref] [ Google Scholar]
- Rabti H, Mohammed Salmani JM, Elamin ES, Lammari N, Zhang J, Ping Q. Carbamazepine solubility enhancement in tandem with swellable polymer osmotic pump tablet: a promising approach for extended delivery of poorly water-soluble drugs. Asian J Pharm Sci 2014; 9(3):146-54. doi: 10.1016/j.ajps.2014.04.001 [Crossref] [ Google Scholar]
- Xu WJ, Li N, Gao CK. Preparation of controlled porosity osmotic pump tablets for salvianolic acid and optimization of the formulation using an artificial neural network method. Acta Pharm Sin B 2011; 1(1):64-70. doi: 10.1016/j.apsb.2011.04.002 [Crossref] [ Google Scholar]
- Maleki Dizaj S, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl 2014; 44:278-84. doi: 10.1016/j.msec.2014.08.031 [Crossref] [ Google Scholar]
- Sahoo CK, Rao SR, Sudhakar M. Evaluation of controlled porosity osmotic pump tablets: a review. Res J Pharm Technol 2015; 8(12):1340-6. doi: 10.5958/0974-360x.2015.00312.1 [Crossref] [ Google Scholar]
- Zakeri-Milani P, Valizadeh H, Tajerzadeh H, Azarmi Y, Islambolchilar Z, Barzegar S. Predicting human intestinal permeability using single-pass intestinal perfusion in rat. J Pharm Pharm Sci 2007; 10(3):368-79. [ Google Scholar]
- Singh K, Walia MK, Agarwal G, Harikumar SL. Osmotic pump drug delivery system: a noval approach. J Drug Deliv Ther 2013; 3(5):156-62. doi: 10.22270/jddt.v3i5.636 [Crossref] [ Google Scholar]
- Javadzadeh Y, Siahi-Shadbad MR, Barzegar-Jalali M, Nokhodchi A. Enhancement of dissolution rate of piroxicam using liquisolid compacts. Farmaco 2005; 60(4):361-5. doi: 10.1016/j.farmac.2004.09.005 [Crossref] [ Google Scholar]
- Ma L, Deng L, Chen J. Applications of poly(ethylene oxide) in controlled release tablet systems: a review. Drug Dev Ind Pharm 2014; 40(7):845-51. doi: 10.3109/03639045.2013.831438 [Crossref] [ Google Scholar]
- Patil PB, Uphade KB, Saudagar RB. A review: osmotic drug delivery system. Pharma Science Monitor 2018; 9(2):283-300. [ Google Scholar]
- Babu CA, Rao MP, Ratna JV. Controlled-porosity osmotic pump tablets-an overview. Asian J Pharm Res Health Care 2010; 2(1):114-26. [ Google Scholar]
- Nokhodchi A, Javadzadeh Y, Siahi-Shadbad MR, Barzegar-Jalali M. The effect of type and concentration of vehicles on the dissolution rate of a poorly soluble drug (indomethacin) from liquisolid compacts. J Pharm Pharm Sci 2005; 8(1):18-25. [ Google Scholar]
- Patel H, Parikh VP. An overview of osmotic drug delivery system: an update review. Int J Bioassays 2017; 6(7):5426-36. doi: 10.21746/ijbio.2017.07.001 [Crossref] [ Google Scholar]
- Shokri J, Nokhodchi A, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, Barzegar-Jalali M. The effect of surfactants on the skin penetration of diazepam. Int J Pharm 2001; 228(1-2):99-107. doi: 10.1016/s0378-5173(01)00805-5 [Crossref] [ Google Scholar]
- Mene HR, Mene NR, Parakh DR, Ingale TB, Magar DR, Mangale MR. Formulation aspects in development of controlled porosity osmotic pump tablet. Pharm Biol Eval 2016; 3(1):1-18. [ Google Scholar]
- Shokri J, Ahmadi P, Rashidi P, Shahsavari M, Rajabi-Siahboomi A, Nokhodchi A. Swellable elementary osmotic pump (SEOP): an effective device for delivery of poorly water-soluble drugs. Eur J Pharm Biopharm 2008; 68(2):289-97. doi: 10.1016/j.ejpb.2007.06.006 [Crossref] [ Google Scholar]
- Keraliya RA, Patel C, Patel P, Keraliya V, Soni TG, Patel RC. Osmotic drug delivery system as a part of modified release dosage form. ISRN Pharm 2012; 2012:528079. doi: 10.5402/2012/528079 [Crossref] [ Google Scholar]
- Shokri J, Adibkia K. Application of cellulose and cellulose derivatives in pharmaceutical industries. In: van de Ven T, Godbout L, eds. Cellulose-Medical, Pharmaceutical and Electronic Applications. IntechOpen; 2013. 10.5772/55178.
- Buchmann S, Sandmann GH, Walz L, Hoppe H, Beitzel K, Wexel G. Refixation of the supraspinatus tendon in a rat model--influence of continuous growth factor application on tendon structure. J Orthop Res 2013; 31(2):300-5. doi: 10.1002/jor.22211 [Crossref] [ Google Scholar]
- Tuntikulwattana S, Mitrevej A, Kerdcharoen T, Williams DB, Sinchaipanid N. Development and optimization of micro/nanoporous osmotic pump tablets. AAPS PharmSciTech 2010; 11(2):924-35. doi: 10.1208/s12249-010-9446-4 [Crossref] [ Google Scholar]
- Davis SS, Hardy JG, Newman SP, Wilding IR. Gamma scintigraphy in the evaluation of pharmaceutical dosage forms. Eur J Nucl Med 1992; 19(11):971-86. doi: 10.1007/bf00175865 [Crossref] [ Google Scholar]
- Liu L, Xu X. Preparation of bilayer-core osmotic pump tablet by coating the indented core tablet. Int J Pharm 2008; 352(1-2):225-30. doi: 10.1016/j.ijpharm.2007.10.047 [Crossref] [ Google Scholar]
- Banerjee A, Verma PR, Gore S. Controlled porosity solubility modulated osmotic pump tablets of gliclazide. AAPS PharmSciTech 2015; 16(3):554-68. doi: 10.1208/s12249-014-0246-0 [Crossref] [ Google Scholar]
- Li G, Wang Y, Chen H, Leng D, Ma P, Dong Y. Can semipermeable membranes coating materials influence in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet: in vitro evaluation and in vivo pharmacokinetics study. Asian J Pharm Sci 2015; 10(2):128-37. doi: 10.1016/j.ajps.2014.12.002 [Crossref] [ Google Scholar]
- Makhija SN, Vavia PR. Controlled porosity osmotic pump-based controlled release systems of pseudoephedrine I Cellulose acetate as a semipermeable membrane. J Control Release 2003; 89(1):5-18. doi: 10.1016/s0168-3659(02)00482-0 [Crossref] [ Google Scholar]
- Abrahamsson B, Alpsten M, Bake B, Jonsson UE, Eriksson-Lepkowska M, Larsson A. Drug absorption from nifedipine hydrophilic matrix extended-release (ER) tablet-comparison with an osmotic pump tablet and effect of food. J Control Release 1998; 52(3):301-10. doi: 10.1016/s0168-3659(97)00267-8 [Crossref] [ Google Scholar]
- Bathool A, Gowda DV, Khan MS, Ahmed A, Vasudha SL, Rohitash K. Development and evaluation of microporous osmotic tablets of diltiazem hydrochloride. J Adv Pharm Technol Res 2012; 3(2):124-9. doi: 10.4103/2231-4040.97292 [Crossref] [ Google Scholar]
- Piyush G, Pankaj R, Dabeer A, Ayaj A. A review on osmotically regulated devices. Int J Pharm Life Sci 2010; 1(6):302-12. [ Google Scholar]
- Shah N, Patel N, Patel K, Patel D. A review on osmotically controlled oral drug delivery systems. J Pharm Sci Biosci Res 2012; 2(5):230-7. [ Google Scholar]
- Kurakula M, Rao G. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): as excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J Drug Deliv Sci Technol 2020; 60:102046. doi: 10.1016/j.jddst.2020.102046 [Crossref] [ Google Scholar]
- Pope DG, Wilkinson PK, Egerton JR, Conroy J. Oral controlled-release delivery of ivermectin in cattle via an osmotic pump. J Pharm Sci 1985; 74(10):1108-10. doi: 10.1002/jps.2600741021 [Crossref] [ Google Scholar]
- Chen J, Pan H, Ye T, Liu D, Li Q, Chen F. Recent aspects of osmotic pump systems: functionalization, clinical use and advanced imaging technology. Curr Drug Metab 2016; 17(3):279-91. doi: 10.2174/1389200216666151015115706 [Crossref] [ Google Scholar]
- Pavani JK, Pavani S, Kumar YS, Venkatesh A, Rao YM. Formulation and evaluation of oral elementary osmotic pump tablets of sumatriptan succinate. J Pharm Res Int 2014; 4(10):1163-73. [ Google Scholar]
- Eckenhoff B, Yum SI. The osmotic pump: novel research tool for optimizing drug regimens. Biomaterials 1981; 2(2):89-97. doi: 10.1016/0142-9612(81)90005-3 [Crossref] [ Google Scholar]
- Kan SL, Li J, Liu JP, Zhao Y. Preparation and IVIVC evaluation of salvianolic acid B micro-porous osmotic pump pellets. Drug Dev Ind Pharm 2015; 41(3):476-81. doi: 10.3109/03639045.2013.879722 [Crossref] [ Google Scholar]
- Liu X, Wang S, Chai L, Zhang D, Sun Y, Xu L. A two-step strategy to design high bioavailable controlled-release nimodipine tablets: the push-pull osmotic pump in combination with the micronization/solid dispersion techniques. Int J Pharm 2014; 461(1-2):529-39. doi: 10.1016/j.ijpharm.2013.12.023 [Crossref] [ Google Scholar]
- Zhang W, Zhang L, Qu X, Zhu Z, Pan Y, Guan J. In vitro and in vivo evaluations of a novel pulsed and controlled osmotic pump capsule. Drug Dev Ind Pharm 2015; 41(2):322-32. doi: 10.3109/03639045.2013.859265 [Crossref] [ Google Scholar]
- Debotton N, Dahan A. Applications of polymers as pharmaceutical excipients in solid oral dosage forms. Med Res Rev 2017; 37(1):52-97. doi: 10.1002/med.21403 [Crossref] [ Google Scholar]
- Ghaffari S, Saleh Vesal M, Jafari Azar Z, Kobarfard F. Preparation and evaluation of osmotic pump systems of soft gelatin capsules, L-OROS softcap, of ibuprofen. Int J Pharm Res Dev 2012; 4(10):9-18. [ Google Scholar]
- Patel A, Mehta T, Patel J, Patel M, Patel K, Patel N. Recent advances in asymmetric membrane capsule based osmotic pump: a patent overview. Recent Pat Drug Deliv Formul 2012; 6(1):66-72. doi: 10.2174/187221112799219099 [Crossref] [ Google Scholar]
- Fara JW. [35] Osmotic delivery systems for research. Methods Enzymol 1985; 112:470-84. doi: 10.1016/S0076-6879(85)12037-9 [Crossref] [ Google Scholar]
- Sotthivirat S, Haslam JL, Stella VJ. Controlled porosity-osmotic pump pellets of a poorly water-soluble drug using sulfobutylether-beta-cyclodextrin, (SBE) 7M-beta-CD, as a solubilizing and osmotic agent. J Pharm Sci 2007; 96(9):2364-74. doi: 10.1002/jps.20891 [Crossref] [ Google Scholar]
- Verma RK, Garg S. Development and evaluation of osmotically controlled oral drug delivery system of glipizide. Eur J Pharm Biopharm 2004; 57(3):513-25. doi: 10.1016/j.ejpb.2004.02.003 [Crossref] [ Google Scholar]
- Yadav AR, Mohite SK. Different techniques and characterization of polymorphism with their evaluation: a review. Asian J Pharm Technol 2020; 10(3):213-6. doi: 10.5958/2231-5713.2020.00035.5 [Crossref] [ Google Scholar]
- Dasankoppa FS, Ningangowdar M, Sholapur H. Formulation and evaluation of controlled porosity osmotic pump for oral delivery of ketorolac. J Basic Clin Phar 2012; 4(1):2-9. doi: 10.4103/0976-0105.109398 [Crossref] [ Google Scholar]
- Wu C, Zhao Z, Zhao Y, Hao Y, Liu Y, Liu C. Preparation of a push-pull osmotic pump of felodipine solubilized by mesoporous silica nanoparticles with a core-shell structure. Int J Pharm 2014; 475(1-2):298-305. doi: 10.1016/j.ijpharm.2014.08.033 [Crossref] [ Google Scholar]
- Sahoo CK, Rao SR, Sudhakar M. Development and evaluation of controlled porosity osmotic pump tablets for zidovudine and lamivudine combination. Res J Pharm Technol 2017; 10(8):2591-601. doi: 10.5958/0974-360x.2017.00460.7 [Crossref] [ Google Scholar]
- Chen J, Wang XC, Liu LX. Preparation of monolithic osmotic pump tablet of poorly soluble atenolol. Chin Pharm J 2008; 43(9):680-2. [ Google Scholar]
- James Stephen P. Development and Evaluation of an Oral Push-Pull Osmotic Pump Tablet of Losartan Potassium [dissertation]. Chennai: CL Baid Mehta College of Pharmacy; 2012.
- Kunz W, Benhabiles A, Ben-Aïm R. Osmotic evaporation through macroporous hydrophobic membranes: a survey of current research and applications. J Memb Sci 1996; 121(1):25-36. doi: 10.1016/0376-7388(96)00153-6 [Crossref] [ Google Scholar]
- Pujara ND, Thacker AP, Dudhat KR, Patel NV, Parmar RB. Osmotically controlled oral drug delivery systems: a novel approach. Inventi Rapid NDDS 2012; 2012(4):1-8. [ Google Scholar]
- Kan SL, Li J, Liu JP, He HL, Zhang WJ. Evaluation of pharmacokinetics and pharmacodynamics relationships for salvianolic acid B micro-porous osmotic pump pellets in angina pectoris rabbit. Asian J Pharm Sci 2014; 9(3):137-45. doi: 10.1016/j.ajps.2014.04.003 [Crossref] [ Google Scholar]
- Shirole PU, Patil PB, Bachhav RS. Review on osmotic drug delivery system. Int J Res Anal Rev 2020; 7(2):7-22. [ Google Scholar]
- Sahoo CK, Rao SR, Sudhakar M. Development and evaluation of controlled release formulation of lamivudine based on microporous osmotic tablet technology using fructose as osmogen. Indonesian J Pharm 2017; 28(3):168-78. doi: 10.14499/indonesianjpharm28iss3pp167 [Crossref] [ Google Scholar]
- Abdul Khaleq NM, Ali WK, Elkordy AA. Effect of coating method on release of glimepiride from porosity osmotic pump tablets (POPTs). Al Mustansiriyah J Pharm Sci 2020; 20(2):37-45. doi: 10.32947/ajps.v20i2.696 [Crossref] [ Google Scholar]