Biomed Res Bull. 1(4):154-159.
doi: 10.34172/brb.2023.29
Review Article
The Relationship of Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Stimulator of the Interferon Gene Pathway With Other Immunity Signaling Pathways in Apoptosis
Navid Shomali 1, * 
Author information:
1Department of Immunology, Faculty of Medicine,Tabriz University of Medical Sciences,Tabriz-Iran
Abstract
Natural immunity, the first defensive mechanism of the body against pathogen invasion, relies on nucleic acid recognition systems to detect the presence of pathogens. The cyclic GMP–AMP synthase-stimulator of interferon (IFN) gene (cGAS-STING) signaling pathway is a crucial pattern recognition and effector pathway in the innate immune system. Its primary function is to detect DNA molecules in the cytoplasm and initiate downstream signaling pathways, resulting in the production of type I IFNs and other inflammatory factors. STING, a pivotal transmembrane protein in the innate immune system, plays a vital role in the host’s ability to resist invasion by foreign pathogens. An increasing amount of evidence suggests that the cGAS-STING pathway induces apoptosis in addition to stimulating inflammatory responses and producing type I IFN. Many previous studies have so far focused on investigating the mechanisms of apoptosis induced by the cGAS-STING pathway as well as the effects that ensue. The relationship between the cGAS-STING pathway and apoptosis has been extensively examined in this article. Through endoplasmic reticulum stress, nucleotide oligomerization domain-like receptor protein-3, nuclear factor-κB (NF-κB), IFN regulatory factor 3, and IFN signals, the cGAS-STING pathway may cause apoptosis. Conversely, apoptosis could affect how the cGAS-STING pathway is regulated. It may release mitochondrial DNA to boost the process or activate caspases to suppress it. The cGAS-STING pathway plays a critical role in controlling innate immune responses, fighting off infections, and stopping the growth of tumors by stopping apoptosis.
Keywords: Apoptosis, cGAS-STING, Immunity
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Stimulator of interferon gene (STING), sometimes referred to as a stimulator of interferon (IFN) genes, MITA, MPYS, or ERIS, is widely distributed in nonlymphoid tissues, including the lung and heart, and is found in hematopoietic cells in peripheral lymphoid organs. STING is localized to the mitochondria-associated endoplasmic reticulum membrane and the endoplasmic reticulum.1 In reaction to foreign DNA originating from several intracellular infections, STING combines with IFN regulatory factor 3 (IRF3) to start the synthesis of type I IFN.2,3 The onset of systemic lupus erythematosus has been linked to STING 4–7. Following its purification and identification, cGAS has emerged as a prominent subject of study in the area of immunology, focusing on its structure and function. The cGAS protein is a member of the nucleotidyltransferase (NTase) superfamily, capable of detecting nucleic acids that are either released from external sources or produced internally.7-9 cGAS is composed of two primary structural domains, including an N-terminal domain with a positive charge that helps stabilize the dimer form of cGAS and a C-terminal domain with a globular structure. The C-terminal domain has two subdomains, including an NTase core, which is responsible for the enzymatic activity of cGAS, and a Mab21 domain, which is involved in binding to DNA.10-13 The Mab21 domain consists of two distinct lobes separated by a deep gap, which is necessary for the binding of nucleotides.14,15 The C-terminal lobe is a bundle of helices with a conserved zinc (Zn) thumb, while the N-terminal lobe is made up of β-sheets encircled by α-helices. The side chains of the active sites of cGAS are oriented toward the groove dividing the N and C lobes, and side chains are located on the central β-sheet of the NTase domain.16-19 Porcine cGAS has decreased catalytic activity when Glu200 and Asp202 are substituted with Gln and Asn, respectively.19-21 In human cGAS, substituting alanine for Gly212, Ser213, Glu225, Asp227, and Asp319 also results in a decrease in catalytic activity.8 Previous research has shown the critical function that the conserved Zn thumb plays in cGAS dimerization and DNA binding processes.22-24 When the cGAS C-terminal domain binds to Zn, the H(X5)CC(X6)C Zn-binding motif, which is inserted between residues 389 and 405, triggers a structural rearrangement. The existence of charged loops that alter the required gap for DNA binding is made possible by the Zn ligand sites.25 This narrative review aims to provide a succinct overview of the current procedures and results related to cyclic GMP–AMP synthase-stimulator of IFN gene (cGAS-STING)-triggered apoptosis.
Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Stimulator of Interferon Gene Pathway Relation With Nucleotide Oligomerization Domain-like Receptor Protein-3 in Apoptosis
Through the induction of caspase-1 activation and the production of proinflammatory cytokines interleukin(IL)-1β/IL-18 in response to microbial infection and cellular damage, NLRP3 performs a critical function in the innate immune system 26. In addition to causing transcription factors IRF3 and nuclear factor-κB (NF-κB) to become active, activating STING also activates the NLRP3 inflammasome.27 It is proposed that the interaction between STING and NLRP3 is the mechanism through which the cGAS-STING pathway activates the NLRP3 inflammasome.28 Through NLRP3 localization and NLRP3 polyubiquitination removal, STING interacts with NLRP3 to facilitate inflammasome activation.28
Furthermore, it was shown that STING may lessen NLRP3’s K48- and K63-linked polyubiquitination after a herpes simplex virus-1 infection or stimulation by cytosolic DNA. The PYRIN domain (PYD), NACHT-associated domain (NAD), and leucine-rich repeat (LRR) domain make up the NLRP3 protein. The results of the tests demonstrated that the interaction with STING included the NAD and LRR domains. Five putative transmembrane segments make up STING. TM5, which is composed of 151–160 amino acids in humans, interacts with NLRP3. Conversely, TM2, which spans amino acids 41–81 in humans, has a role in the NLRP3 inflammasome’s formation and activation.29 Moreover, an independent study has revealed that the cGAS-STING pathway may damage lysosomes and cause potassium ions to be released, which might activate the NLRP3 inflammasome.29 When BLaER1 monocytes were stimulated with DNA, the amount of potassium (K + ) inside the cells decreased significantly, and this reduction was dependent on the cGAS-STING pathway.30
Additionally, an increasing number of studies have reported that NLRP3 inflammasome activation contributes to several types of cell death, including apoptosis, in addition to pyroptosis.30 The direct enzyme caspase-8 converts IL-1β when the NLRP3 inflammasome is activated. NLRP3-apoptosis-associated speck-like protein inflammasomes attract caspase-8 when they are active, which helps murine bone marrow-derived dendritic cells process IL-1β. This procedure takes place regardless of the existence of caspase-1 and caspase-11.31 If the pyroptotic process is inhibited but the inflammasome is activated, caspase-8 may act as a substitute mechanism and cause apoptosis.32 NLRP3 inflammasomes directly exploit caspase-8 as a substantial IL-1β-converting protease and as a pro-apoptotic initiator when caspase-1 is absent.32 Thus, it may be concluded that by activating the NLRP3 pathway, the cGAS-STING pathway can cause apoptosis (Figure 1). A mouse investigation revealed that STING activated NLRP3, which, in turn, caused inflammation and apoptosis in the heart. Cardiomyocyte apoptosis and NLRP3-mediated inflammation were successfully inhibited by STING silencing.33
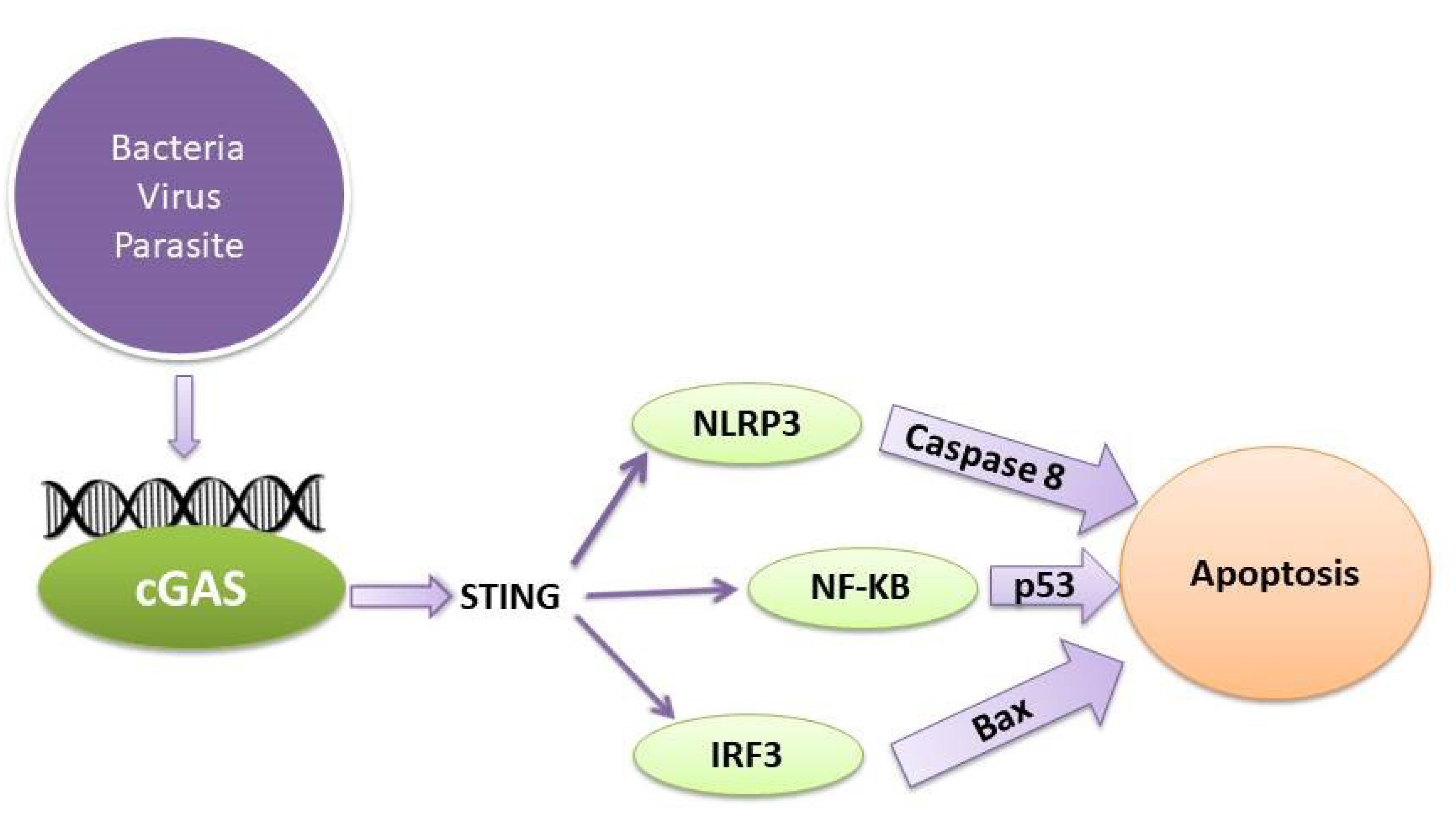
Figure 1.
The Relationship of the cGAS-STING Pathway With Apoptosis. Note. cGAS-STING: Cyclic GMP–AMP synthase-stimulator of interferon gene
.
The Relationship of the cGAS-STING Pathway With Apoptosis. Note. cGAS-STING: Cyclic GMP–AMP synthase-stimulator of interferon gene
Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Stimulator of Interferon Gene Pathway Relation With NF-κB in Apoptosis
The production of cytokines is regulated by NF-κB, a critical regulator of inflammatory immune responses.34 Research has shown that the cGAS-STING pathway might trigger NF-κB, which would then cause the inflammatory response to start. This mechanism may also help the immune system fight off infections.35,36 The transcription of genes that encode inflammatory cytokines may be regulated by the cGAS-STING pathway via the induction of NF-κB-dependent signaling transduction. TBK1 and its homolog IκB kinase epsilon (IKKε) trigger the IKK complex, which, in turn, activates the transcription factor NF-κB after being stimulated by STING.37 STING activates IKK during endoplasmic reticulum translocation and is phosphorylated at Ser374 in humans (and Ser373 in mice). This process causes IκB to become phosphorylated, which is then followed by the ubiquitin-proteasome system destroying it and releasing free NF-κB.38 Moreover, STING causes the Golgi apparatus’s IKK complex to become active, causing the unbound NF-κB to go into the nucleus.39
Although NF-κB is well known for its anti-apoptotic function, which prevents cell death, it is also often observed that pro-apoptotic effects, which cause cell death, may result from NF-κB activation.40 Through the activation of several anti-apoptotic genes, NF-κB negatively affects apoptosis. Apoptosis occurs if NF-κB activity is inhibited.41 Nonetheless, NF-κB activation may enhance the beginning of programmed cell death in human osteosarcoma cells by upregulating the expression of the protein known as Bcl-2-binding component 3, or p53-upregulated modulator of apoptosis.42 Additionally, studies have demonstrated that NF-κB may cause apoptosis by triggering the synthesis of genes that promote apoptosis.43 STING can initiate the NF-κB signaling pathway. Conversely, inhibiting NF-κB signaling by the use of siRNA p65 reduces the effects of STING-induced apoptosis and senescence improving the imbalance in STING-induced extracellular matrix metabolism.44 Further, ultraviolet B (UVB) may trigger programmed cell death (apoptosis) in human keratinocyte (HaCaT) cells via activating the cGAS-STING pathway. The administration of BAY, a substance that inhibits the NF-κB pathway, can prevent UVB-induced cell death. Therefore, the NF-κB signal plays a role in the induction of apoptosis via the cGAS-STING pathway (Figure 1).
Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Stimulator of Interferon Gene Pathway Relation With Interferon-I in Apoptosis
The cGAS-STING signaling pathway detects viral infections and triggers the synthesis of type 1 IFNs to counteract the invading pathogens. Studies have revealed that type 1 IFNs may trigger apoptosis in different cell types using both intrinsic and extrinsic pathways.45-47 IFN-β has been shown to trigger apoptosis in neuroblastoma cells by inhibiting the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathway, releasing cytochrome c, and activating procaspase 9 via the intrinsic route.48 Nevertheless, a separate investigation demonstrated that the initiation of programmed cell death (apoptosis) by IFN-β relies on the activation of caspase-8 via the extrinsic route.49-52 Furthermore, it was observed that the apoptosis triggered by IFN-β may be hindered by specific inhibitors that target caspase-8 but not caspase-9.53-55 Likewise, several investigations have shown that IFN-I promotes apoptosis via the extrinsic signaling route. This mechanism also relies on the presence of the death ligand TRAIL in melanoma and breast cancer cells.56 Thus, the generation of IFN-I may be involved in the mechanism of apoptosis triggered by the cGAS-STING pathway (Figure 1).
IFN-I controls apoptosis through a process that involves many signaling pathways. Among these pathways, the Janus kinase/signal transducers and activators of transcription (JAK-STAT) and PI3K/AKT pathways are thought to play important roles.52 According to some theories, IFN-I might cause apoptosis by triggering the IFN-JAK-STAT pathway, which controls the Bcl-2 family of proteins.52 After binding to IFN-α/β receptors, IFN-I phosphorylates and activates Tyk2 and JAK1, two JAK family members. These kinases then go on to phosphorylate STAT1. It was shown that STAT1 activation was involved in the control of apoptosis. This was accomplished by regulating the ERK1/2 and JNK pathways to inhibit the activity of Bcl-2 and Bax, two Bcl-2 family members.57 In addition, IFN-1 may cause apoptosis by blocking the PI3K/AKT signaling pathway. Serine/threonine protein kinases belonging to the PI3K family are the building blocks of the PI3K/AKT signaling pathway. It is essential for inhibiting apoptosis and promoting cell division.58
Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Stimulator of Interferon Gene Pathway Relation With Phosphatidylinositol 3-Kinase in Apoptosis
PI3K plays a crucial role in the growth and specialization of B lymphocytes. The class I PI3K is composed of two parts, namely, a catalytic subunit called p110 and a regulatory subunit called p85.59 The absence of p110δ in mice leads to a notable decrease in splenic marginal zone B cells and B1a cells.60,61 Mice lacking phosphatase and tensin homolog specifically in B cells have heightened PI3K signaling, along with augmented populations of marginal zone B cells and B1a cells.62-65 The lack of phosphatase and tensin homolog and the resulting elevation in PI-3,4-P2 and PI-3,4,5-P3 may serve as a replacement for CD19 in enhancing PI3K activity.62 Another inositol phosphatase, Src homology 2 domain-containing inositol 5′-phosphatase (SHIP), also demonstrates a similar function. Lyn catalyzes the phosphorylation of CD19, which subsequently recruits PI3Ks. The PI3K/PIP3 signaling pathway activates AKT signaling via AKT/PDK-1 activation, leading to a decrease in apoptosis. This is achieved by phosphorylating Foxo-1/3, which promotes their export from the nucleus and subsequent destruction.65,66 PIP3 interacts with PDK1, leading to the phosphorylation of AKT. This phosphorylation, either directly or indirectly, activates the mammalian target of rapamycin complex 1 by involving tuberous sclerosis complex 1/tuberous sclerosis complex 1.67,68 STING has shown the ability to control the activity of the tyrosine phosphatase SHP.69 However, it is yet unknown if STING can also regulate SHIP during B-cell receptor activation.
Conclusion
Apoptosis could affect how the cGAS-STING pathway is regulated. It may release mitochondrial DNA to boost the process or activate caspases to suppress it. The cGAS-STING pathway plays a critical role in controlling innate immune responses, fighting off infections, and stopping the growth of tumors by stopping apoptosis.
Acknowledgments
I would like to thank the Department of Immunology at Tabriz University of Medical Sciences.
Competing Interests
The author declares no conflict of interests.
Ethical Approval
Not applicable.
Funding
The study received no financial support.
References
- Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol 2016; 17(10):1142-9. doi: 10.1038/ni.3558 [Crossref] [ Google Scholar]
- Pandey S, Kawai T, Akira S. Microbial sensing by toll-like receptors and intracellular nucleic acid sensors. Cold Spring Harb Perspect Biol 2014; 7(1):a016246. doi: 10.1101/cshperspect.a016246 [Crossref] [ Google Scholar]
- Zhang X, Wu J, Du F, Xu H, Sun L, Chen Z. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Rep 2014; 6(3):421-30. doi: 10.1016/j.celrep.2014.01.003 [Crossref] [ Google Scholar]
- Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 2013; 39(6):1019-31. doi: 10.1016/j.immuni.2013.10.019 [Crossref] [ Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013; 339(6121):786-91. doi: 10.1126/science.1232458 [Crossref] [ Google Scholar]
- Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008; 455(7213):674-8. doi: 10.1038/nature07317 [Crossref] [ Google Scholar]
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009; 461(7265):788-92. doi: 10.1038/nature08476 [Crossref] [ Google Scholar]
- Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe 2015; 18(2):157-68. doi: 10.1016/j.chom.2015.07.001 [Crossref] [ Google Scholar]
- Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal 2012; 5(214):ra20. doi: 10.1126/scisignal.2002521 [Crossref] [ Google Scholar]
- Saitoh T, Fujita N, Hayashi T, Takahara K, Satoh T, Lee H. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A 2009; 106(49):20842-6. doi: 10.1073/pnas.0911267106 [Crossref] [ Google Scholar]
- Mukai K, Konno H, Akiba T, Uemura T, Waguri S, Kobayashi T. Activation of STING requires palmitoylation at the Golgi. Nat Commun 2016; 7:11932. doi: 10.1038/ncomms11932 [Crossref] [ Google Scholar]
- Taguchi T, Mukai K, Takaya E, Shindo R. STING operation at the ER/Golgi interface. Front Immunol 2021; 12:646304. doi: 10.3389/fimmu.2021.646304 [Crossref] [ Google Scholar]
- Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol 2015; 15(12):760-70. doi: 10.1038/nri3921 [Crossref] [ Google Scholar]
- Guerriero JL. Macrophages: their untold story in T cell activation and function. Int Rev Cell Mol Biol 2019; 342:73-93. doi: 10.1016/bs.ircmb.2018.07.001 [Crossref] [ Google Scholar]
- Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology 2018; 154(2):186-95. doi: 10.1111/imm.12910 [Crossref] [ Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8(12):958-69. doi: 10.1038/nri2448 [Crossref] [ Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41(1):14-20. doi: 10.1016/j.immuni.2014.06.008 [Crossref] [ Google Scholar]
- Li Q, Liu C, Yue R, El-Ashram S, Wang J, He X. cGAS/STING/TBK1/IRF3 signaling pathway activates BMDCs maturation following Mycobacterium bovis infection. Int J Mol Sci 2019; 20(4):895. doi: 10.3390/ijms20040895 [Crossref] [ Google Scholar]
- Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014; 41(5):830-42. doi: 10.1016/j.immuni.2014.10.017 [Crossref] [ Google Scholar]
- Li T, Chen ZJ. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J Exp Med 2018; 215(5):1287-99. doi: 10.1084/jem.20180139 [Crossref] [ Google Scholar]
- Farhood B, Najafi M, Mortezaee K. CD8( + ) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol 2019; 234(6):8509-21. doi: 10.1002/jcp.27782 [Crossref] [ Google Scholar]
- Kurachi M. CD8( + ) T cell exhaustion. Semin Immunopathol 2019; 41(3):327-37. doi: 10.1007/s00281-019-00744-5 [Crossref] [ Google Scholar]
- Henning AN, Roychoudhuri R, Restifo NP. Epigenetic control of CD8( + ) T cell differentiation. Nat Rev Immunol 2018; 18(5):340-56. doi: 10.1038/nri.2017.146 [Crossref] [ Google Scholar]
- Dimeloe S, Burgener AV, Grählert J, Hess C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology 2017; 150(1):35-44. doi: 10.1111/imm.12655 [Crossref] [ Google Scholar]
- Zhu J. T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb Perspect Biol 2018; 10(10):a030338. doi: 10.1101/cshperspect.a030338 [Crossref] [ Google Scholar]
- Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol 2015; 33:445-74. doi: 10.1146/annurev-immunol-032414-112043 [Crossref] [ Google Scholar]
- Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov 2020; 10(1):26-39. doi: 10.1158/2159-8290.cd-19-0761 [Crossref] [ Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 2009; 138(2):271-85. doi: 10.1016/j.cell.2009.05.046 [Crossref] [ Google Scholar]
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138(2):286-99. doi: 10.1016/j.cell.2009.05.045 [Crossref] [ Google Scholar]
- Rendtlew Danielsen JM, Knudsen LM, Dahl IM, Lodahl M, Rasmussen T. Dysregulation of CD47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br J Haematol 2007; 138(6):756-60. doi: 10.1111/j.1365-2141.2007.06729.x [Crossref] [ Google Scholar]
- Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015; 21(10):1209-15. doi: 10.1038/nm.3931 [Crossref] [ Google Scholar]
- Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 2015; 16(9):907-17. doi: 10.1038/ni.3253 [Crossref] [ Google Scholar]
- Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol 2018; 36:489-517. doi: 10.1146/annurev-immunol-042617-053010 [Crossref] [ Google Scholar]
- Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol 2015; 33:355-91. doi: 10.1146/annurev-immunol-032414-112103 [Crossref] [ Google Scholar]
- Zhou Y, Fei M, Zhang G, Liang WC, Lin W, Wu Y, et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. immunity 2020;52(2):357-73.e9. 10.1016/j.immuni.2020.01.014.
- Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity 2012; 37(6):986-97. doi: 10.1016/j.immuni.2012.09.014 [Crossref] [ Google Scholar]
- Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A 2011; 108(42):17396-401. doi: 10.1073/pnas.1113421108 [Crossref] [ Google Scholar]
- Cunha LD, Yang M, Carter R, Guy C, Harris L, Crawford JC, et al. LC3-associated phagocytosis in myeloid cells promotes tumor immune tolerance. Cell 2018;175(2):429-41.e16. 10.1016/j.cell.2018.08.061.
- Wang C, Guan Y, Lv M, Zhang R, Guo Z, Wei X, et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity 2018;48(4):675-87.e7. 10.1016/j.immuni.2018.03.017.
- Lv M, Chen M, Zhang R, Zhang W, Wang C, Zhang Y. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res 2020; 30(11):966-79. doi: 10.1038/s41422-020-00395-4 [Crossref] [ Google Scholar]
- Jing W, McAllister D, Vonderhaar EP, Palen K, Riese MJ, Gershan J. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer 2019; 7(1):115. doi: 10.1186/s40425-019-0573-5 [Crossref] [ Google Scholar]
- Klarquist J, Hennies CM, Lehn MA, Reboulet RA, Feau S, Janssen EM. STING-mediated DNA sensing promotes antitumor and autoimmune responses to dying cells. J Immunol 2014; 193(12):6124-34. doi: 10.4049/jimmunol.1401869 [Crossref] [ Google Scholar]
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014; 41(5):843-52. doi: 10.1016/j.immuni.2014.10.019 [Crossref] [ Google Scholar]
- Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A 2015; 112(50):15408-13. doi: 10.1073/pnas.1512832112 [Crossref] [ Google Scholar]
- Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 2015; 7(283):283ra52. doi: 10.1126/scitranslmed.aaa4306 [Crossref] [ Google Scholar]
- Ohkuri T, Kosaka A, Ishibashi K, Kumai T, Hirata Y, Ohara K. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol Immunother 2017; 66(6):705-16. doi: 10.1007/s00262-017-1975-1 [Crossref] [ Google Scholar]
- Qi Z, Yan F, Chen D, Xing W, Li Q, Zeng W. Identification of prognostic biomarkers and correlations with immune infiltrates among cGAS-STING in hepatocellular carcinoma. Biosci Rep 2020; 40(10):BSR20202603. doi: 10.1042/bsr20202603 [Crossref] [ Google Scholar]
- Cheng N, Watkins-Schulz R, Junkins RD, David CN, Johnson BM, Montgomery SA. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight 2018; 3(22):e120638. doi: 10.1172/jci.insight.120638 [Crossref] [ Google Scholar]
- Chen YP, Xu L, Tang TW, Chen CH, Zheng QH, Liu TP. STING activator c-di-GMP-loaded mesoporous silica nanoparticles enhance immunotherapy against breast cancer. ACS Appl Mater Interfaces 2020; 12(51):56741-52. doi: 10.1021/acsami.0c16728 [Crossref] [ Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 2015; 11(7):1018-30. doi: 10.1016/j.celrep.2015.04.031 [Crossref] [ Google Scholar]
- Wilski NA, Stotesbury C, Del Casale C, Montoya B, Wong E, Sigal LJ. STING sensing of murine cytomegalovirus alters the tumor microenvironment to promote antitumor immunity. J Immunol 2020; 204(11):2961-72. doi: 10.4049/jimmunol.1901136 [Crossref] [ Google Scholar]
- Yu J, Deng H, Xu Z. Targeting macrophage priming by polyphyllin VII triggers anti-tumor immunity via STING-governed cytotoxic T-cell infiltration in lung cancer. Sci Rep 2020; 10(1):21360. doi: 10.1038/s41598-020-77800-w [Crossref] [ Google Scholar]
- Gadkaree SK, Fu J, Sen R, Korrer MJ, Allen C, Kim YJ. Induction of tumor regression by intratumoral STING agonists combined with anti-programmed death-L1 blocking antibody in a preclinical squamous cell carcinoma model. Head Neck 2017; 39(6):1086-94. doi: 10.1002/hed.24704 [Crossref] [ Google Scholar]
- Temizoz B, Kuroda E, Ohata K, Jounai N, Ozasa K, Kobiyama K. TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. Eur J Immunol 2015; 45(4):1159-69. doi: 10.1002/eji.201445132 [Crossref] [ Google Scholar]
- Kocabas BB, Almacioglu K, Bulut EA, Gucluler G, Tincer G, Bayik D. Dual-adjuvant effect of pH-sensitive liposomes loaded with STING and TLR9 agonists regress tumor development by enhancing Th1 immune response. J Control Release 2020; 328:587-95. doi: 10.1016/j.jconrel.2020.09.040 [Crossref] [ Google Scholar]
- Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41(1):49-61. doi: 10.1016/j.immuni.2014.06.010 [Crossref] [ Google Scholar]
- Zhang Y, Sun Z, Pei J, Luo Q, Zeng X, Li Q. Identification of α-mangostin as an agonist of human STING. ChemMedChem 2018; 13(19):2057-64. doi: 10.1002/cmdc.201800481 [Crossref] [ Google Scholar]
- Downey CM, Aghaei M, Schwendener RA, Jirik FR. DMXAA causes tumor site-specific vascular disruption in murine non-small cell lung cancer, and like the endogenous non-canonical cyclic dinucleotide STING agonist, 2’3’-cGAMP, induces M2 macrophage repolarization. PLoS One 2014; 9(6):e99988. doi: 10.1371/journal.pone.0099988 [Crossref] [ Google Scholar]
- Ohkuri T, Kosaka A, Nagato T, Kobayashi H. Effects of STING stimulation on macrophages: STING agonists polarize into “classically” or “alternatively” activated macrophages?. Hum Vaccin Immunother 2018; 14(2):285-7. doi: 10.1080/21645515.2017.1395995 [Crossref] [ Google Scholar]
- Shevtsov M, Sato H, Multhoff G, Shibata A. Novel approaches to improve the efficacy of immuno-radiotherapy. Front Oncol 2019; 9:156. doi: 10.3389/fonc.2019.00156 [Crossref] [ Google Scholar]
- Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 2013; 39(3):482-95. doi: 10.1016/j.immuni.2013.08.004 [Crossref] [ Google Scholar]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017; 8:15618. doi: 10.1038/ncomms15618 [Crossref] [ Google Scholar]
- Liu Y, Crowe WN, Wang L, Lu Y, Petty WJ, Habib AA. An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long-term control of lung metastases. Nat Commun 2019; 10(1):5108. doi: 10.1038/s41467-019-13094-5 [Crossref] [ Google Scholar]
- Zierhut C, Yamaguchi N, Paredes M, Luo JD, Carroll T, Funabiki H. The cytoplasmic DNA sensor cGAS promotes mitotic cell death. Cell 2019;178(2):302-15.e23. 10.1016/j.cell.2019.05.035.
- Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019; 38(13):2380-93. doi: 10.1038/s41388-018-0581-9 [Crossref] [ Google Scholar]
- Ghaffari A, Peterson N, Khalaj K, Vitkin N, Robinson A, Francis JA. STING agonist therapy in combination with PD-1 immune checkpoint blockade enhances response to carboplatin chemotherapy in high-grade serous ovarian cancer. Br J Cancer 2018; 119(4):440-9. doi: 10.1038/s41416-018-0188-5 [Crossref] [ Google Scholar]
- Ethiraj P, Veerappan K, Samuel S, Sivapatham S. Interferon β improves the efficacy of low dose cisplatin by inhibiting NF-κB/p-Akt signaling on HeLa cells. Biomed Pharmacother 2016; 82:124-32. doi: 10.1016/j.biopha.2016.04.058 [Crossref] [ Google Scholar]
- Li Q, Kawamura K, Yang S, Okamoto S, Kobayashi H, Tada Y. Interferon-β produces synergistic combinatory anti-tumor effects with cisplatin or pemetrexed on mesothelioma cells. PLoS One 2013; 8(8):e72709. doi: 10.1371/journal.pone.0072709 [Crossref] [ Google Scholar]
- Li T, Cheng H, Yuan H, Xu Q, Shu C, Zhang Y. Antitumor activity of cGAMP via stimulation of cGAS-cGAMP-STING-IRF3 mediated innate immune response. Sci Rep 2016; 6:19049. doi: 10.1038/srep19049 [Crossref] [ Google Scholar]