Biomed Res Bull. 1(3):105-108.
doi: 10.34172/biomedrb.2023.20
Original Article
The Effects of CAR T-cell Therapy on Interleukin 6 and Interleukin 1 Inflammatory Cytokines, and TLR2 and TLR4 Expression in Patients With Dental Implants Who Received the COVID-19 Vaccine
Mohaddeseh Rajabpour 1  , Farshad Anguti 1, Mobina Belalzadeh 1, Ali Mohebbi 1, *
, Farshad Anguti 1, Mobina Belalzadeh 1, Ali Mohebbi 1, * 
Author information:
1Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
COVID-19 is a family of coronaviruses that was started in Wuhan, China in 2020. This virus has a strong relationship with cytokine storm. The aim of this novel study was to evaluate the chimeric antigen receptor (CAR) T-cell therapy drug’s effects on immunity member expression in dental implant patients who received the COVID-19 vaccine.
Methods:
A total of ten patients who received the COVID-19 vaccine and applied for dental implants were chosen for this study. Five patients who applied for dental implants but did not receive the COVID-19 vaccine were selected as the control group. The macrophage cells were isolated from dental pulp and treated with CAR T-cell therapy drugs. The real-time polymerase chain reaction was performed for the evaluation of Toll-like receptor 2 (TLR2) and TLR4 expression, and an enzyme-linked immunosorbent assay was used to determine the levels of interleukin (IL-6) and IL-1 cytokines.
Results:
The results of the present study showed that 48 hours after treating the cells with CAR T-cell therapy drugs, the TLR2 and TLR4 expression and IL-6 and IL-1 levels were decreased, and this amount was higher in the tisagenlecleucel group than in the axicabtagene ciloleucel group (P<0.001). The COVID-19 vaccine group results demonstrated no significant difference compared with other groups.
Conclusion:
The findings of the present study proved that the drugs as a CAR T-cell therapy, such as Tisagenlecleucel and Axicabtagene ciloleucel, could decrease TLR function and inflammatory cytokines levels, but due to the cytokines storm in COVID-19, these drugs could not affect innate immunity receptors and cytokines levels.
Keywords: CAR T-cell, IL-6, IL-1
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a beta coronavirus that causes pneumonia.1. The virus attacks the respiratory system and causes dyspnea, fever, dry cough, weakness, vomiting, diarrhea, hypoxia, and the like.2 The SARS-CoV-2 virus activates the immune response and causes inflammatory reactions that lead to the release of cytokines in the body of the infected person. These cytokines cause immune abnormalities leading to infections by microorganisms, septic shock, and severe multiple organ dysfunction.3 Pro-inflammatory cytokines, such as interleukin (IL)-6, are found at high concentrations in severe patient bodies.4 Dental implants are an integrated system of technology developed in collaboration with various disciplines, including chemistry, surface physics, and biomechanics. There are extremely strict requirements and controls for making implants. These materials have biological and mechanical compatibility with the human body since they are in contact with the soft and hard tissues of the body. These implants are made of titanium, which is used in adults to restore health and beauty. Dental implants are surgically placed under the gums. The bone begins to grow around and inside the implant, creating strong support that rests on a superstructure.5 The function of chimeric antigen receptors (CARs) is to direct immune cells to identify and target tumor cell surface molecules. CAR cell therapy has received a great deal of attention as immunotherapy and has received the approval of the Food and Drug Administration. These cells are less tissue-compatible in the treatment of tumors. These cells are unsuitable for long-term therapy since they develop toxicity after administration.6 The human body has two types of immune responses to germs, including innate and adaptive immunity. The mechanism of action of both of these responses is to distinguish between internal and external molecules. The innate response has cellular receptors called Toll-like receptors (TLR) that detect microbe-specific molecules. These receptors produce specific signals that trigger an inflammatory response when they come in contact with germs. Hence, TLRs are important components of the innate safety pass.7 IL-1, IL-6, and tumor necrosis factor are three examples of multifunctional cytokines that play an important role in the immune response, hematopoiesis, and inflammation. Each of them has a specific task, but in general, their function is to interact with each other. IL-6 has the task of inducing antibodies. IL-6 belongs to the cytokine receptor family, while IL-1 belongs to the immunoglobulin family.8 With upper respiratory tract infection by COVID-19, mild respiratory syndrome occurs with the release of IL-1β and IL-6. The binding of the virus to the TLR releases pro-IL-1β. The enzyme caspase 1 cleaves this cytokine, and then inflammatory activation occurs and IL-1β is produced, which is the main cause of pneumonia, fever, and fibrosis. Thus, the suppression of IL-1 and IL-6 reduces inflammation.9 This study aims to determine how CAR T-cells affect innate immunity cytokines and receptors in patients with the COVID-19 vaccination who applied for an implant.
Materials and Methods
Patients and Cell Culture and Treatment
A total of ten patients who received the COVID-19 vaccine and applied for dental implants were selected for this study. Five patients who applied for dental implants while not receiving the COVID-19 vaccine were chosen as the control group. The macrophage cells were isolated and cultured according to a previous study 10. The cells were treated with tisagenlecleucel (Tisa-Cel) and axicabtagene ciloleucel (Yescarta) within 2, 12, 24, 48, and 72 hours.
Real-Time Polymerase Chain Reaction
The cells were cultured in a 6-well plate. After treatment with CAR T-cell therapy drugs, total RNAs were extracted by the TRIzol reagent based on the manufacturer’s kit protocol (Takara, Japan), and mRNA was extracted. Quantitative RT-PCR was applied to measure the expression levels of TLR2 and TLR4. It should be noted that all samples were controlled by β-actin.
Enzyme-Linked Immunosorbent Assay
The supernatant of the cells was collected and and IL-1 and IL-6 were evaluated by ELISA kit (R&D System, USA). The detection threshold of the assay was found to be 1 ng/mL.
Western Blot
The treated and untreated macrophage cells were lysed by RIPA Lysis Buffer (Sigma-Aldrich, Germany). For electrophoresis, 50 μg of the extracted protein was added to the gel. Polyvinylidene difluoride membranes were used for staining the proteins. The cells were blocked, and the membrane was incubated with anti-TLR2, anti-TLR4, and beta-actin (Sigma-Aldrich, Germany). The density of proteins was measured using ImageJ software.
Statically Analysis
The data were summarized as means ± standard deviations (SD). The statistical analyses were performed by the independent-sample t test, and P values< 0.05 were statistically significant.
Results
Toll-Like Receptors 2 and 4 Expression Decreased With Chimeric Antigen Receptor T-cell Therapy
The macrophage cells were isolated from dental pulp and treated with CAR T-cell drugs. The time course of RT-PCR results demonstrated lower expression of TLR2 and TLR4 in drug groups compared with the control group. The results showed that in groups treated with Tisagenlecleucel, TLR2 and TLR4 expressions decreased significantly in comparison to Axicabtagene ciloleucel groups. In addition, TLR2 expression decreased more than TLR4. Further, in both TLR2 and TLR4 groups, the expression decreased significantly after 48 hours compared with other time groups (Figure 1A). The western blot was performed for the evaluation of TLR2 and TLR4 protein expression. Similar to the results of RT-PCR, the results revealed lower expression of TLR2 and TLR4 in the Tisagenlecleucel group (P< 0.001, Figure 1B).
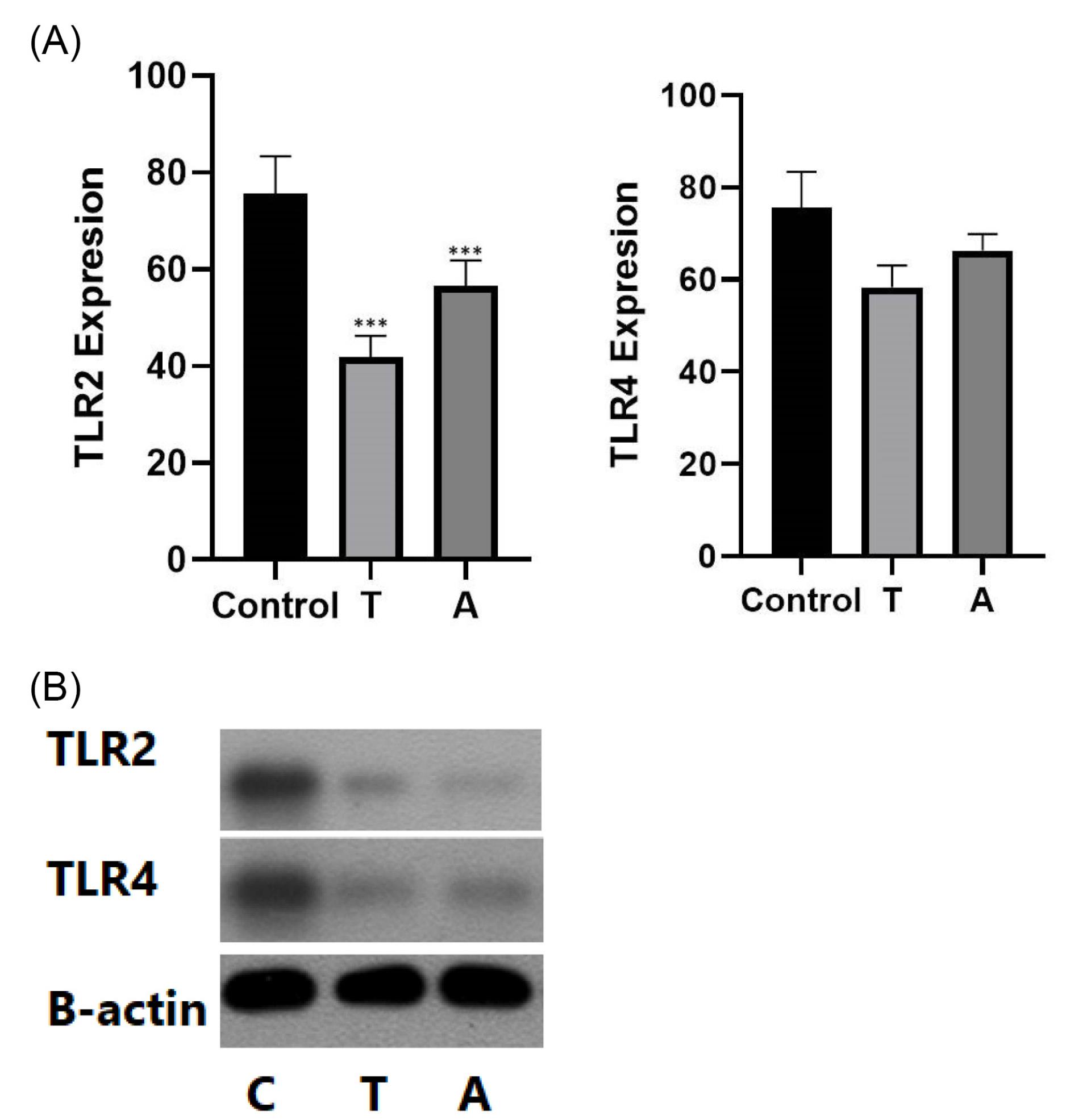
Figure 1.
(A) The TLR2 and TLR4 Expression in Both Vaccine Groups. (B) The TLR2 and TLR4 Protein Expression in Both Vaccine Groups. Note. TLR: Toll-like receptor
.
(A) The TLR2 and TLR4 Expression in Both Vaccine Groups. (B) The TLR2 and TLR4 Protein Expression in Both Vaccine Groups. Note. TLR: Toll-like receptor
Interleukin 6 and Interleukin 1 Levels Decreased With Chimeric Antigen Receptor T-cell Therapy
The supernatant of macrophage cells was collected for the measurement of IL-6 and IL-1 levels. The levels were detected via ELISA. Based on the results, the levels of IL-6 and IL-1 decreased significantly in the Tisagenlecleucel group compared with the Axicabtagene ciloleucel groups. Furthermore, the results showed that the cytokine levels decreased within 48 hours compared to other time courses.
The ELISA results for IL-6 demonstrated that the level of IL-6 cytokine decreased significantly compared to IL-1 (Figure 2).
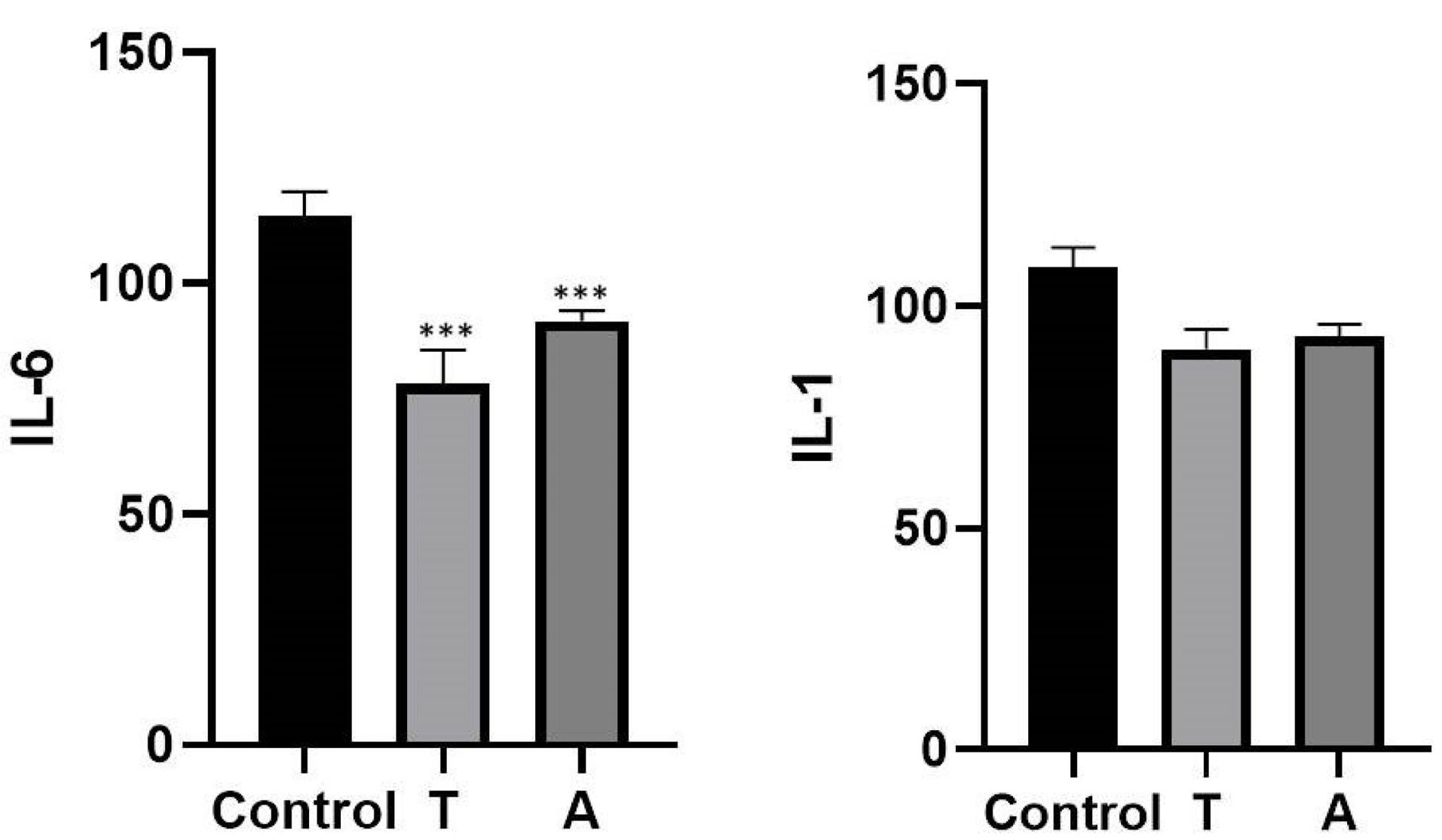
Figure 2.
Determination of the IL-1 and IL-6 Levels via ELISA. Note. IL: Interleukin; ELISA: Enzyme-linked immunosorbent assay
.
Determination of the IL-1 and IL-6 Levels via ELISA. Note. IL: Interleukin; ELISA: Enzyme-linked immunosorbent assay
Discussion
Many studies have focused on CAR T-cells that mediate major histocompatibility complex-unrestricted in tumor cell killing by enabling T cells to bind target cell surface antigens, but a few studies have addressed CAR T-cell therapy in dentistry subjects.11 Tisagenlecleucel is a CD19-directed genetically modified autologous T-cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory follicular lymphoma after two or more lines of therapy. Axicabtagene ciloleucel is not recommended for the treatment of patients with primary central nervous system lymphoma. CAR T-cell therapy has an important relationship with innate immunity, such as cytokines and receptors.12 Recent studies have shown that CAR T-cell therapy drugs such asTisagenlecleucel decrease the innate immunity receptor function in tumor cells, but no study has so far focused on these functions in dental pulps.13,14 Our results revealed that the CAR T-cell therapy drugs can affect TLRs and decrease their function. This is a novel study that has focused on the different effects of CAR T-cell therapy drugs. Likewise, the studies reported that these drugs can decrease inflammation during cancer, but few studies have addressed dental pulps.15 The present study results confirmed that CAR T-cell therapy drugs decrease the levels of pro-inflammatory cytokines such as IL-6 and IL-1. The current study focused on patients who received the COVID-19 vaccines, and the results showed that the CAR T-cell therapy drugs could not decrease the receptor’s function or levels of inflammatory cytokines in these groups compared with other groups (Figures 1 and 2). As mentioned earlier, the present study is a novel study on dentistry subjects, hoping to help researchers follow and increase these subjects’ data. The present study’s results proved that drugs used as CAR T-cell therapy, such as Tisagenlecleucel and Axicabtagene ciloleucel, could decrease TLR function and inflammatory cytokines levels; however, due to cytokines storm in COVID-19, these drugs could not affect innate immunity receptors and cytokines levels.
Conclusion
The findings of the present study proved that the drugs as a CAR T-cell therapy, such as Tisagenlecleucel and Axicabtagene ciloleucel, could decrease TLR function and inflammatory cytokines levels, but due to the cytokines storm in COVID-19, these drugs could not affect innate immunity receptors and cytokines levels.
Author’s Contribution
Conceptualization: Mohaddeseh Rajabpour.
Data curation: Frashad Anguti, Mobina Belalzadeh, Ali Mohebbi.
Formal analysis: Ali Mohebbi.
Funding acquisition: Ali Mohebbi.
Investigation: Frashad Anguti, Mobina Belalzadeh, Ali Mohebbi.
Methodology: Frashad Anguti, Mobina Belalzadeh.
Project administration: Frashad Anguti, Mobina Belalzadeh.
Software: Mobina Belalzadeh.
Supervision: Ali Mohebbi.
Writing–original draft: Frashad Anguti, Mobina Belalzadeh.
Writing–review & editing: Ali Mohebbi.
Competing Interests
None.
Ethical Approval
The ethical was approved by Tabriz University of medical sciences committee (Code: IR.IAU.TABRIZ.REC.1395.4).
Funding
None.
References
- Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang CB, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci 2020; 57(6):365-88. doi: 10.1080/10408363.2020.1783198 [Crossref] [ Google Scholar]
- Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol 2020; 215:108427. doi: 10.1016/j.clim.2020.108427 [Crossref] [ Google Scholar]
- Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther 2020; 5(1):128. doi: 10.1038/s41392-020-00243-2 [Crossref] [ Google Scholar]
- Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol 2020; 11:1708. doi: 10.3389/fimmu.2020.01708 [Crossref] [ Google Scholar]
- Oshida Y, Tuna EB, Aktören O, Gençay K. Dental implant systems. Int J Mol Sci 2010; 11(4):1580-678. doi: 10.3390/ijms11041580 [Crossref] [ Google Scholar]
- Kankeu Fonkoua LA, Sirpilla O, Sakemura R, Siegler EL, Kenderian SS. CAR T cell therapy and the tumor microenvironment: current challenges and opportunities. Mol Ther Oncolytics 2022; 25:69-77. doi: 10.1016/j.omto.2022.03.009 [Crossref] [ Google Scholar]
- Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol 2011; 21(13):R488-93. doi: 10.1016/j.cub.2011.05.039 [Crossref] [ Google Scholar]
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J 1990; 4(11):2860-7. [ Google Scholar]
- Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVID-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020; 34(2):327-31. doi: 10.23812/conti-e [Crossref] [ Google Scholar]
- Li R, Pei S, Chen B, Song Y, Zhang T, Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020; 368(6490):489-93. doi: 10.1126/science.abb3221 [Crossref] [ Google Scholar]
- Liu D, Zhao J, Song Y. Engineering switchable and programmable universal CARs for CAR T therapy. J Hematol Oncol 2019; 12(1):69. doi: 10.1186/s13045-019-0763-0 [Crossref] [ Google Scholar]
- Chen Z, Xiong H, Li JX, Li H, Tao F, Yang YT. [COVID-19 with post-chemotherapy agranulocytosis in childhood acute leukemia: a case report]. Zhonghua Xue Ye Xue Za Zhi 2020; 41(4):341-3. doi: 10.3760/cma.j.issn.0253-2727.2020.0004.[Chinese] [Crossref] [ Google Scholar]
- Asghari Ozma M, Maroufi P, Khodadadi E, Köse Ş, Esposito I, Ganbarov K. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez Med 2020; 28(2):153-65. [ Google Scholar]
- Liu D, Zhao J. Cytokine release syndrome: grading, modeling, and new therapy. J Hematol Oncol 2018; 11(1):121. doi: 10.1186/s13045-018-0653-x [Crossref] [ Google Scholar]
- Yakoub-Agha I, Chabannon C, Bader P, Basak GW, Bonig H, Ciceri F. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica 2020; 105(2):297-316. doi: 10.3324/haematol.2019.229781 [Crossref] [ Google Scholar]